Abstract
Exercise, stress, sleep and diet are four distinct but intertwined lifestyle factors that influence the cardiovascular system. Abundant epidemiological, clinical and preclinical studies have underscored the importance of managing stress, having good sleep hygiene and responsible eating habits and exercising regularly. We are born with a genetic blueprint that can protect us against or predispose us to a particular disease. However, lifestyle factors build upon and profoundly influence those predispositions. Studies in the past 10 years have shown that the immune system in general and leukocytes in particular are particularly susceptible to environmental perturbations. Lifestyle factors such as stress, sleep, diet and exercise affect leukocyte behaviour and function and thus the immune system at large. In this Review, we explore the various mechanisms by which lifestyle factors modulate haematopoiesis and leukocyte migration and function in the context of cardiovascular health. We pay particular attention to the role of the nervous system as the key executor that connects environmental influences to leukocyte behaviour.
Key points
-
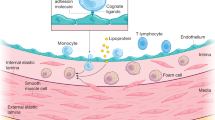
Cardiovascular disease (CVD), and atherosclerosis in particular, is driven by chronic underlying inflammation, leading to plaque destabilization by the infiltration of leukocytes.
-
The risk of CVD is only partly driven by genetic predisposition; the exposome, consisting of environmental and personal factors, has an important role in the inflammatory progression of CVD.
-
Modification of lifestyle factors such as exercise, stress, sleep patterns and diet holds the potential to help in both reducing disease burden and revealing the effects that these factors have on the immune system.
-
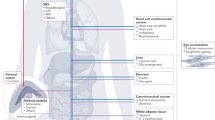
Findings from the past decade highlight the connection between the hypothalamic–pituitary–adrenal axis and the sympathetic nervous system, and modifiable lifestyle factors and the cardiovascular system.
-
Improving our understanding of the influence of the nervous system on the exposome, as well as on CVD and the immune system, can potentially help to lower CVD burden and its complications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lloyd-Jones, D. M. et al. Life’s essential 8: updating and enhancing the American Heart Association’s Construct of Cardiovascular Health: a presidential advisory from the American Heart Association. Circulation 146, e18–e43 (2022).
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 Study. J. Am. Coll. Cardiol. 76, 2982–3021 (2020).
Libby, P. et al. Atherosclerosis. Nat. Rev. Dis. Prim. 5, 56 (2019).
Engelen, S. E., Robinson, A. J. B., Zurke, Y. X. & Monaco, C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed. Nat. Rev. Cardiol. 19, 522–542 (2022).
Libby, P., Ridker, P. M. & Hansson, G. K. Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317–325 (2011).
Nidorf, S. M. et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 383, 1838–1847 (2020).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Tardif, J. C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505 (2019).
Mendelson, A. & Frenette, P. S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 20, 833–846 (2014).
Libby, P. The changing landscape of atherosclerosis. Nature 592, 524–533 (2021).
Swirski, F. K. & Nahrendorf, M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339, 161–166 (2013).
Mindur, J. E. & Swirski, F. K. Growth factors as immunotherapeutic targets in cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 39, 1275–1287 (2019).
Stiekema, L. C. A. et al. Impact of cholesterol on proinflammatory monocyte production by the bone marrow. Eur. Heart J. 42, 4309–4320 (2021).
van der Valk, F. M. et al. Increased haematopoietic activity in patients with atherosclerosis. Eur. Heart J. 38, 425–432 (2017).
Courties, G. et al. Glucocorticoids regulate bone marrow B lymphopoiesis after stroke. Circ. Res. 124, 1372–1385 (2019).
Heidt, T. et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 20, 754–758 (2014).
Mendez-Ferrer, S. & Frenette, P. S. Hematopoietic stem cell trafficking: regulated adhesion and attraction to bone marrow microenvironment. Ann. N. Y. Acad. Sci. 1116, 392–413 (2007).
de Juan, A. et al. Artery-associated sympathetic innervation drives rhythmic vascular inflammation of arteries and veins. Circulation 140, 1100–1114 (2019).
Wang, C., Lutes, L. K., Barnoud, C. & Scheiermann, C. The circadian immune system. Sci. Immunol. 7, eabm2465 (2022).
Mohanta, S. K. et al. Neuroimmune cardiovascular interfaces control atherosclerosis. Nature 605, 152–159 (2022).
Trott, D. W. et al. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 64, 1108–1115 (2014).
Marvar, P. J. & Harrison, D. G. Stress-dependent hypertension and the role of T lymphocytes. Exp. Physiol. 97, 1161–1167 (2012).
Bowers, E. & Singer, K. Obesity-induced inflammation: the impact of the hematopoietic stem cell niche. JCI Insight 6, e145295 (2021).
Nagareddy, P. R. et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 19, 821–835 (2014).
Osborn, O. & Olefsky, J. M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 18, 363–374 (2012).
Rappaport, S. M. Implications of the exposome for exposure science. J. Expo. Sci. Environ. Epidemiol. 21, 5–9 (2011).
Turner, M. C. et al. Assessing the exposome with external measures: commentary on the state of the science and research recommendations. Annu. Rev. Public Health 38, 215–239 (2017).
Yu, E., Malik, V. S. & Hu, F. B. Cardiovascular disease prevention by diet modification: JACC health promotion series. J. Am. Coll. Cardiol. 72, 914–926 (2018).
Dong, T. A. et al. Intermittent fasting: a heart healthy dietary pattern? Am. J. Med. 133, 901–907 (2020).
Horne, B. D. et al. Usefulness of routine periodic fasting to lower risk of coronary artery disease in patients undergoing coronary angiography. Am. J. Cardiol. 102, 814–819 (2008).
Sutton, E. F. et al. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 27, 1212–1221.e3 (2018).
Rong, S. et al. Association of skipping breakfast with cardiovascular and all-cause mortality. J. Am. Coll. Cardiol. 73, 2025–2032 (2019).
Jordan, S. et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell 178, 1102–1114 e1117 (2019).
Janssen, H. et al. Monocytes re-enter the bone marrow during fasting and alter the host response to infection. Immunity 56, 783–796.e7 (2023).
Collins, N. et al. The bone marrow protects and optimizes immunological memory during dietary restriction. Cell 178, 1088–1101.e15 (2019).
Nagai, M. et al. Fasting-refeeding impacts immune cell dynamics and mucosal immune responses. Cell 178, 1072–1087.e14 (2019).
Chen, Y. et al. Intermittent fasting inhibits high-fat diet-induced atherosclerosis by ameliorating hypercholesterolemia and reducing monocyte chemoattraction. Front. Pharmacol. 12, 719750 (2021).
Okoshi, K. et al. Influence of intermittent fasting on myocardial infarction-induced cardiac remodeling. BMC Cardiovasc. Disord. 19, 126 (2019).
Makimura, H. et al. Role of glucocorticoids in mediating effects of fasting and diabetes on hypothalamic gene expression. BMC Physiol. 3, 5 (2003).
La Rose, A. M. et al. Hepatocyte-specific glucose-6-phosphatase deficiency disturbs platelet aggregation and decreases blood monocytes upon fasting-induced hypoglycemia. Mol. Metab. 53, 101265 (2021).
Chawla, S., Beretoulis, S., Deere, A. & Radenkovic, D. The window matters: a systematic review of time restricted eating strategies in relation to cortisol and melatonin secretion. Nutrients 13, 2525 (2021).
Chan, J. L., Mietus, J. E., Raciti, P. M., Goldberger, A. L. & Mantzoros, C. S. Short-term fasting-induced autonomic activation and changes in catecholamine levels are not mediated by changes in leptin levels in healthy humans. Clin. Endocrinol. 66, 49–57 (2007).
Poller, W. C. et al. Brain motor and fear circuits regulate leukocytes during acute stress. Nature 607, 578–584 (2022).
Liu, T. et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron 73, 511–522 (2012).
Landry, T. et al. Energy status differentially modifies feeding behavior and POMC(ARC) neuron activity after acute treadmill exercise in untrained mice. Front. Endocrinol. 12, 705267 (2021).
Matarese, G. et al. Hunger-promoting hypothalamic neurons modulate effector and regulatory T-cell responses. Proc. Natl Acad. Sci. USA 110, 6193–6198 (2013).
Douglass, A. M. et al. Neural basis for fasting activation of the hypothalamic–pituitary–adrenal axis. Nature 620, 154–162 (2023).
Kivimaki, M. & Steptoe, A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 15, 215–229 (2018).
Roozendaal, B., McEwen, B. S. & Chattarji, S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 10, 423–433 (2009).
Visseren, F. L. J. et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 42, 3227–3337 (2021).
Hinterdobler, J., Schunkert, H., Kessler, T. & Sager, H. B. Impact of acute and chronic psychosocial stress on vascular inflammation. Antioxid. Redox Signal. 35, 1531–1550 (2021).
Osborne, M. T. et al. Disentangling the links between psychosocial stress and cardiovascular disease. Circ. Cardiovasc. Imaging 13, e010931 (2020).
Wilbert-Lampen, U. et al. Cardiovascular events during World Cup soccer. N. Engl. J. Med. 358, 475–483 (2008).
Hinterdobler, J. et al. Acute mental stress drives vascular inflammation and promotes plaque destabilization in mouse atherosclerosis. Eur. Heart J. 42, 4077–4088 (2021).
Goebel, M. U., Mills, P. J., Irwin, M. R. & Ziegler, M. G. Interleukin-6 and tumor necrosis factor-alpha production after acute psychological stress, exercise, and infused isoproterenol: differential effects and pathways. Psychosom. Med. 62, 591–598 (2000).
Vaccarino, V. et al. Association of mental stress-induced myocardial ischemia with cardiovascular events in patients with coronary heart disease. JAMA 326, 1818–1828 (2021).
Breen, M. S. et al. Acute psychological stress induces short-term variable immune response. Brain Behav. Immun. 53, 172–182 (2016).
Hammadah, M. et al. Inflammatory response to mental stress and mental stress induced myocardial ischemia. Brain Behav. Immun. 68, 90–97 (2018).
Marsland, A. L., Walsh, C., Lockwood, K. & John-Henderson, N. A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav. Immun. 64, 208–219 (2017).
Moazzami, K. et al. Higher activation of the rostromedial prefrontal cortex during mental stress predicts major cardiovascular disease events in individuals with coronary artery disease. Circulation 142, 455–465 (2020).
Lagraauw, H. M., Wezel, A., van der Velden, D., Kuiper, J. & Bot, I. Stress-induced mast cell activation contributes to atherosclerotic plaque destabilization. Sci. Rep. 9, 2134 (2019).
Xu, C., Lee, S. K., Zhang, D. & Frenette, P. S. The gut microbiome regulates psychological-stress-induced inflammation. Immunity 53, 417–428.e4 (2020).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Hodes, G. E. et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl Acad. Sci. USA 111, 16136–16141 (2014).
Qing, H. et al. Origin and function of stress-induced IL-6 in murine models. Cell 182, 372–387.e14 (2020).
Osborne, M. T. et al. The combined effect of air and transportation noise pollution on atherosclerotic inflammation and risk of cardiovascular disease events. J. Nucl. Cardiol. 30, 665–679 (2023).
Munzel, T., Sorensen, M. & Daiber, A. Transportation noise pollution and cardiovascular disease. Nat. Rev. Cardiol. 18, 619–636 (2021).
Rosengren, A. et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case–control study. Lancet 364, 953–962 (2004).
von Kanel, R., Bellingrath, S. & Kudielka, B. M. Association between burnout and circulating levels of pro- and anti-inflammatory cytokines in schoolteachers. J. Psychosom. Res. 65, 51–59 (2008).
Marvar, P. J. et al. T lymphocytes and vascular inflammation contribute to stress-dependent hypertension. Biol. Psychiatry 71, 774–782 (2012).
Joels, M. & Baram, T. Z. The neuro-symphony of stress. Nat. Rev. Neurosci. 10, 459–466 (2009).
Arnsten, A. F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 10, 410–422 (2009).
Tawakol, A. et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 389, 834–845 (2017).
Kang, D. O. et al. Stress-associated neurobiological activity is linked with acute plaque instability via enhanced macrophage activity: a prospective serial 18F-FDG-PET/CT imaging assessment. Eur. Heart J. 42, 1883–1895 (2021).
Sohrabi, Y., Reinecke, H. & Soehnlein, O. Trilateral interaction between innervation, leukocyte, and adventitia: a new driver of atherosclerotic plaque formation. Signal. Transduct. Target. Ther. 7, 249 (2022).
Glaser, R. & Kiecolt-Glaser, J. K. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 5, 243–251 (2005).
Mezue, K. et al. Reduced stress-related neural network activity mediates the effect of alcohol on cardiovascular risk. J. Am. Coll. Cardiol. 81, 2315–2325 (2023).
Schakel, L. et al. Effectiveness of stress-reducing interventions on the response to challenges to the immune system: a meta-analytic review. Psychother. Psychosom. 88, 274–286 (2019).
Grandner, M. A. & Fernandez, F. X. The translational neuroscience of sleep: a contextual framework. Science 374, 568–573 (2021).
Unruh, M. L. et al. Subjective and objective sleep quality and aging in the sleep heart health study. J. Am. Geriatr. Soc. 56, 1218–1227 (2008).
Dominguez, F. et al. Association of sleep duration and quality with subclinical atherosclerosis. J. Am. Coll. Cardiol. 73, 134–144 (2019).
Ford, E. S., Cunningham, T. J. & Croft, J. B. Trends in self-reported sleep duration among US adults from 1985 to 2012. Sleep 38, 829–832 (2015).
Garbarino, S., Lanteri, P., Bragazzi, N. L., Magnavita, N. & Scoditti, E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 4, 1304 (2021).
Cappuccio, F. P., Cooper, D., D’Elia, L., Strazzullo, P. & Miller, M. A. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur. Heart J. 32, 1484–1492 (2011).
Zuraikat, F. M., Wood, R. A., Barragan, R. & St-Onge, M. P. Sleep and diet: mounting evidence of a cyclical relationship. Annu. Rev. Nutr. 41, 309–332 (2021).
Daghlas, I. et al. Sleep duration and myocardial infarction. J. Am. Coll. Cardiol. 74, 1304–1314 (2019).
Wang, Y. H. et al. Association of longitudinal patterns of habitual sleep duration with risk of cardiovascular events and all-cause mortality. JAMA Netw. Open. 3, e205246 (2020).
Soehnlein, O. & Libby, P. Targeting inflammation in atherosclerosis — from experimental insights to the clinic. Nat. Rev. Drug Discov. 20, 589–610 (2021).
Libby, P., Nahrendorf, M. & Swirski, F. K. Monocyte heterogeneity in cardiovascular disease. Semin. Immunopathol. 35, 553–562 (2013).
Poller, W. C., Nahrendorf, M. & Swirski, F. K. Hematopoiesis and cardiovascular disease. Circ. Res. 126, 1061–1085 (2020).
Friedman, G. D., Klatsky, A. L. & Siegelaub, A. B. The leukocyte count as a predictor of myocardial infarction. N. Engl. J. Med. 290, 1275–1278 (1974).
Rothe, G. et al. Peripheral blood mononuclear phagocyte subpopulations as cellular markers in hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 16, 1437–1447 (1996).
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007).
Heyde, A. et al. Increased stem cell proliferation in atherosclerosis accelerates clonal hematopoiesis. Cell 184, 1348–1361.e22 (2021).
Swirski, F. K. & Nahrendorf, M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 18, 733–744 (2018).
Tall, A. R., Yvan-Charvet, L., Westerterp, M. & Murphy, A. J. Cholesterol efflux: a novel regulator of myelopoiesis and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 32, 2547–2552 (2012).
Tall, A. R. & Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104–116 (2015).
Westerterp, M. et al. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell 11, 195–206 (2012).
Yvan-Charvet, L. et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 328, 1689–1693 (2010).
Asada, N., Takeishi, S. & Frenette, P. S. Complexity of bone marrow hematopoietic stem cell niche. Int. J. Hematol. 106, 45–54 (2017).
Robbins, C. S. et al. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 125, 364–374 (2012).
Robbins, C. S. et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 19, 1166–1172 (2013).
McAlpine, C. S. et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 566, 383–387 (2019).
McAlpine, C. S. et al. Sleep exerts lasting effects on hematopoietic stem cell function and diversity. J. Exp. Med. 219, e20220081 (2022).
Fuster, J. J. et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847 (2017).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Jaiswal, S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 377, 111–121 (2017).
Libby, P. & Ebert, B. L. CHIP (clonal hematopoiesis of indeterminate potential): potent and newly recognized contributor to cardiovascular risk. Circulation 138, 666–668 (2018).
Marnell, C. S., Bick, A. & Natarajan, P. Clonal hematopoiesis of indeterminate potential (CHIP): linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J. Mol. Cell Cardiol. 161, 98–105 (2021).
Steensma, D. P. Clinical consequences of clonal hematopoiesis of indeterminate potential. Blood Adv. 2, 3404–3410 (2018).
Jaiswal, S. & Libby, P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat. Rev. Cardiol. 17, 137–144 (2020).
Zink, F. et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood 130, 742–752 (2017).
Mitchell, E. et al. Clonal dynamics of haematopoiesis across the human lifespan. Nature 606, 343–350 (2022).
Poon, G. Y. P., Watson, C. J., Fisher, D. S. & Blundell, J. R. Synonymous mutations reveal genome-wide levels of positive selection in healthy tissues. Nat. Genet. 53, 1597–1605 (2021).
Pasupuleti, S. K. et al. Obesity-induced inflammation exacerbates clonal hematopoiesis. J. Clin. Invest. 133, e163968 (2023).
Kessler, M. D. et al. Common and rare variant associations with clonal haematopoiesis phenotypes. Nature 612, 301–309 (2022).
Rohde, D. et al. Bone marrow endothelial dysfunction promotes myeloid cell expansion in cardiovascular disease. Nat. Cardiovasc. Res. 1, 28–44 (2022).
Dashti, H. S. et al. Genetic determinants of daytime napping and effects on cardiometabolic health. Nat. Commun. 12, 900 (2021).
Depner, C. M. et al. Ad libitum weekend recovery sleep fails to prevent metabolic dysregulation during a repeating pattern of insufficient sleep and weekend recovery sleep. Curr. Biol. 29, 957–967.e4 (2019).
Leger, D., Richard, J. B., Collin, O., Sauvet, F. & Faraut, B. Napping and weekend catchup sleep do not fully compensate for high rates of sleep debt and short sleep at a population level (in a representative nationwide sample of 12,637 adults). Sleep Med. 74, 278–288 (2020).
Abboud, F. & Kumar, R. Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J. Clin. Invest. 124, 1454–1457 (2014).
Kritikou, I. et al. Sleep apnoea and the hypothalamic–pituitary–adrenal axis in men and women: effects of continuous positive airway pressure. Eur. Respir. J. 47, 531–540 (2016).
Tietjens, J. R. et al. Obstructive sleep apnea in cardiovascular disease: a review of the literature and proposed multidisciplinary clinical management strategy. J. Am. Heart Assoc. 8, e010440 (2019).
Geovanini, G. R. et al. Elevations in neutrophils with obstructive sleep apnea: the Multi-Ethnic Study of Atherosclerosis (MESA). Int. J. Cardiol. 257, 318–323 (2018).
Florido, R. et al. Six-year changes in physical activity and the risk of incident heart failure: ARIC study. Circulation 137, 2142–2151 (2018).
Schroeder, E. C., Franke, W. D., Sharp, R. L. & Lee, D. C. Comparative effectiveness of aerobic, resistance, and combined training on cardiovascular disease risk factors: a randomized controlled trial. PLoS One 14, e0210292 (2019).
Madssen, E. et al. Coronary atheroma regression and plaque characteristics assessed by grayscale and radiofrequency intravascular ultrasound after aerobic exercise. Am. J. Cardiol. 114, 1504–1511 (2014).
Campo, G. et al. Exercise intervention improves quality of life in older adults after myocardial infarction: randomised clinical trial. Heart 106, 1658–1664 (2020).
Gabriel, H., Urhausen, A., Brechtel, L., Muller, H. J. & Kindermann, W. Alterations of regular and mature monocytes are distinct, and dependent of intensity and duration of exercise. Eur. J. Appl. Physiol. Occup. Physiol. 69, 179–181 (1994).
Neves, P. et al. Acute effects of high- and low-intensity exercise bouts on leukocyte counts. J. Exerc. Sci. Fit. 13, 24–28 (2015).
Timmerman, K. L., Flynn, M. G., Coen, P. M., Markofski, M. M. & Pence, B. D. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise. J. Leukoc. Biol. 84, 1271–1278 (2008).
Johannsen, N. M. et al. Effect of different doses of aerobic exercise on total white blood cell (WBC) and WBC subfraction number in postmenopausal women: results from DREW. PLoS ONE 7, e31319 (2012).
Frodermann, V. et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat. Med. 25, 1761–1771 (2019).
Agha, N. H. et al. Vigorous exercise mobilizes CD34+ hematopoietic stem cells to peripheral blood via the beta(2)-adrenergic receptor. Brain Behav. Immun. 68, 66–75 (2018).
Baker, J. M., De Lisio, M. & Parise, G. Endurance exercise training promotes medullary hematopoiesis. FASEB J. 25, 4348–4357 (2011).
De Lisio, M. & Parise, G. Characterization of the effects of exercise training on hematopoietic stem cell quantity and function. J. Appl. Physiol. 113, 1576–1584 (2012).
Aengevaeren, V. L. et al. Relationship between lifelong exercise volume and coronary atherosclerosis in athletes. Circulation 136, 138–148 (2017).
Liao, Z. et al. Early moderate exercise benefits myocardial infarction healing via improvement of inflammation and ventricular remodelling in rats. J. Cell. Mol. Med. 23, 8328–8342 (2019).
Dourida, M. et al. Endocrine responses after a single bout of moderate aerobic exercise in healthy adult humans. J. Appl. Biomed. 17, 46 (2019).
Hill, E. E. et al. Exercise and circulating cortisol levels: the intensity threshold effect. J. Endocrinol. Invest. 31, 587–591 (2008).
Jacks, D. E., Sowash, J., Anning, J., McGloughlin, T. & Andres, F. Effect of exercise at three exercise intensities on salivary cortisol. J. Strength Cond. Res. 16, 286–289 (2002).
Tokinoya, K. et al. Plasma free metanephrine and normethanephrine levels correlated to plasma catecholamine after acute running in amateur runner. J. Exerc. Sci. Fit. 19, 178–181 (2021).
Vasamsetti, S. B. et al. Sympathetic neuronal activation triggers myeloid progenitor proliferation and differentiation. Immunity 49, 93–106.e7 (2018).
Jiang, L. et al. Leptin receptor-expressing neuron Sh2b1 supports sympathetic nervous system and protects against obesity and metabolic disease. Nat. Commun. 11, 1517 (2020).
Fedewa, M. V., Hathaway, E. D., Ward-Ritacco, C. L., Williams, T. D. & Dobbs, W. C. The effect of chronic exercise training on leptin: a systematic review and meta-analysis of randomized controlled trials. Sports Med. 48, 1437–1450 (2018).
Northey, J. M., Cherbuin, N., Pumpa, K. L., Smee, D. J. & Rattray, B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br. J. Sports Med. 52, 154–160 (2018).
Chaddock-Heyman, L. et al. Aerobic fitness is associated with greater hippocampal cerebral blood flow in children. Dev. Cogn. Neurosci. 20, 52–58 (2016).
Erickson, K. I. et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl Acad. Sci. USA 108, 3017–3022 (2011).
Seifert, T. et al. Endurance training enhances BDNF release from the human brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R372–R377 (2010).
Ibeas, K., Herrero, L., Mera, P. & Serra, D. Hypothalamus-skeletal muscle crosstalk during exercise and its role in metabolism modulation. Biochem. Pharmacol. 190, 114640 (2021).
Miletta, M. C. et al. AgRP neurons control compulsive exercise and survival in an activity-based anorexia model. Nat. Metab. 2, 1204–1211 (2020).
He, Z. et al. Cellular and synaptic reorganization of arcuate NPY/AgRP and POMC neurons after exercise. Mol. Metab. 18, 107–119 (2018).
Ben-Shaanan, T. L. et al. Activation of the reward system boosts innate and adaptive immunity. Nat. Med. 22, 940–944 (2016).
Sato, D. et al. Tumor suppression and improvement in immune systems by specific activation of dopamine D1-receptor-expressing neurons in the nucleus accumbens. Mol. Brain 15, 17 (2022).
Zhang, X. et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature 581, 204–208 (2020).
Kerage, D., Sloan, E. K., Mattarollo, S. R. & McCombe, P. A. Interaction of neurotransmitters and neurochemicals with lymphocytes. J. Neuroimmunol. 332, 99–111 (2019).
Felger, J. C. et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 21, 1358–1365 (2016).
Lewitus, G. M., Pribiag, H., Duseja, R., St-Hilaire, M. & Stellwagen, D. An adaptive role of TNFalpha in the regulation of striatal synapses. J. Neurosci. 34, 6146–6155 (2014).
Groot, H. E., van Blokland, I. V., Lipsic, E., Karper, J. C. & van der Harst, P. Leukocyte profiles across the cardiovascular disease continuum: a population-based cohort study. J. Mol. Cell. Cardiol. 138, 158–164 (2020).
Nicolaides, N. C., Vgontzas, A. N., Kritikou, I. & Chrousos, G. HPA axis and sleep. In Endotext (eds Feingold, K. R. et al.) (MDText, 2000).
McStay, M. et al. Intermittent fasting and sleep: a review of human trials. Nutrients 13, 3489 (2021).
Jurado-Fasoli, L. et al. Exercise training improves sleep quality: a randomized controlled trial. Eur. J. Clin. Invest. 50, e13202 (2020).
Acknowledgements
F.K.S. is funded by the National Institutes of Health R35 HL135752, P01 HL131478 and P01 HL142494. H.J. is supported by the German Research Foundation JA 2545/2-1. The authors thank K. Joyes (Cardiovascular Research Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA) for help with editing the manuscript before initial submission.
Author information
Authors and Affiliations
Contributions
The authors contributed substantially to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Daniela Carnevale; Andreas Habenicht, who co-reviewed with Sarajo Mohanta; and Andrew Murphy for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Janssen, H., Koekkoek, L.L. & Swirski, F.K. Effects of lifestyle factors on leukocytes in cardiovascular health and disease. Nat Rev Cardiol 21, 157–169 (2024). https://doi.org/10.1038/s41569-023-00931-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-023-00931-w
This article is cited by
-
Uncovering atherosclerotic cardiovascular disease by PET imaging
Nature Reviews Cardiology (2024)