Abstract
The activation of stress-related neuroendocrine systems helps to maintain homeostasis, but excessive stress can damage body functions. We review current evidence from basic sciences and epidemiology linking stress to the development and progression of metabolic disorders throughout life. Findings from rodents demonstrate that stress can affect features of metabolic dysfunction, such as insulin resistance, glucose and lipid homeostasis, as well as ageing processes such as cellular senescence and telomere length shortening. In human studies, stressors in the home, workplace and neighbourhood are associated with accelerated ageing and metabolic and immune alterations, both directly and indirectly via behavioural risks. The likelihood of developing clinical conditions, such as diabetes mellitus and hepatic steatosis is increased in individuals with adverse childhood experiences or long-term (years) or severe stress at work or in private life. The increased risk of metabolic disorders is often associated with other stress-related conditions, such as mental health disorders, cardiovascular disease and increased susceptibility to infections. Equally, stress can worsen prognosis in metabolic diseases. As favourable modifications in stressors are associated with reductions in incidence of metabolic disorders, further investigation of the therapeutic value of targeting stress in personalized medicine is warranted.
Key points
-
Both animal and human research suggests that stress and related changes in sympathetic–parasympathetic balance and the hypothalamic–pituitary–adrenal axis can accelerate biological ageing, including unfavourable changes in metabolism and immune function.
-
The adverse impact on metabolic disease risk in adults with a history of childhood adversity is potentiated by mental disorders and behavioural risks and the risk of metabolic disease can be more than twofold higher than in adults without childhood adversity.
-
In adults, stress is associated with a 1.1-fold to 1.4-fold excess risk of obesity, diabetes mellitus and liver disease.
-
The excess risk for mental disorders, such as depression, and cardiovascular disease among individuals with stress in adulthood is slightly higher than that for obesity, diabetes mellitus and liver disease.
-
Life stress is also a prognostic factor in patients, accelerating the transition of metabolic diseases towards multimorbidity, frailty and death.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020).
Paik, J. M. et al. The growing burden of disability related to nonalcoholic fatty liver disease: data from the Global Burden of Disease 2007–2017. Hepatol. Commun. 4, 1769–1780 (2020).
Dai, H. et al. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the Global Burden of Disease Study. PLoS Med. 17, e1003198 (2020).
McEwen, B. S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 338, 171–179 (1998).
Koolhaas, J. M. et al. Stress revisited: a critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 35, 1291–1301 (2011). This review of animal models and human studies suggests that stress is characterized by the absence of an anticipatory response (unpredictable) or a reduced recovery (uncontrollable) of the neuroendocrine reaction, and proposes a definition for the term ‘stress’ as a condition where environmental demands exceed the natural regulatory capacity of an organism.
Epel, E. S. et al. More than a feeling: a unified view of stress measurement for population science. Front. Neuroendocrinol. 49, 146–169 (2018).
Snyder-Mackler, N. et al. Social determinants of health and survival in humans and other animals. Science 368, eaax9553 (2020). This paper demonstrates strong parallels in the consequences of social adversity between humans and other social mammals, and reviews studies in experimental animal models that show socially induced stress is, by itself, sufficient to damage health and shorten lifespan.
Bartolomucci, A. Social stress, immune functions and disease in rodents. Front. Neuroendocrinol. 28, 28–49 (2007).
Kirschbaum, C., Pirke, K. M. & Hellhammer, D. H. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiol 28, 76–81 (1993).
Harkness, K. L. & Monroe, S. M. The assessment and measurement of adult life stress: basic premises, operational principles, and design requirements. J. Abnorm. Psychol. 125, 727–745 (2016).
Kivimaki, M. & Steptoe, A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 15, 215–229 (2018).
Cohen, S., Janicki-Deverts, D. & Miller, G. E. Psychological stress and disease. JAMA 298, 1685–1687 (2007).
Lampert, R. et al. Triggering of symptomatic atrial fibrillation by negative emotion. J. Am. Coll. Cardiol. 64, 1533–1534 (2014).
Gunnar, M. & Quevedo, K. The neurobiology of stress and development. Annu. Rev. Psychol. 58, 145–173 (2007).
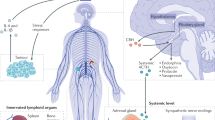
Russell, G. & Lightman, S. The human stress response. Nat. Rev. Endocrinol. 15, 525–534 (2019). A review of evidence on the human stress response, the cortisol ultradian rhythmicity under basal and stressful conditions and their relevance for cardiovascular, immunological and metabolic function.
Ulrich-Lai, Y. M. & Herman, J. P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 10, 397–409 (2009).
Tawakol, A. et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 389, 834–884 (2017).
Lightman, S. L., Birnie, M. T. & Conway-Campbell, B. L. Dynamics of ACTH and cortisol secretion and implications for disease. Endocr. Rev. 41, bnaa002 (2020).
Russell, E., Koren, G., Rieder, M. & Van Uum, S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 37, 589–601 (2012).
Sara, J. D. S. et al. Mental stress and its effects on vascular health. Mayo Clin. Proc. 97, 951–990 (2022).
Razzoli, M. et al. Social stress shortens lifespan in mice. Aging Cell 17, e12778 (2018). A mouse model that demonstrates the adverse effect of subordinate social status on lifespan, implicating cellular senescence in ageing-associated diseases.
Koert, A. et al. The social instability stress paradigm in rat and mouse: a systematic review of protocols, limitations, and recommendations. Neurobiol. Stress. 5, 100410 (2021).
Lacroix, A., Feelders, R. A., Stratakis, C. A. & Nieman, L. K. Cushing’s syndrome. Lancet 386, 913–927 (2015).
Ferrau, F. & Korbonits, M. Metabolic comorbidities in Cushing’s syndrome. Eur. J. Endocrinol. 173, M133–M157 (2015).
Constantinescu, G. et al. Glucocorticoid excess in patients with pheochromocytoma compared with paraganglioma and other forms of hypertension. J. Clin. Endocrinol. Metab. 105, dgaa423 (2020).
O’Donnell, C. J. et al. Posttraumatic stress disorder and cardiovascular disease: state of the science, knowledge gaps, and research opportunities. JAMA Cardiol. 6, 1207–1216 (2021).
Wingenfeld, K., Whooley, M. A., Neylan, T. C., Otte, C. & Cohen, B. E. Effect of current and lifetime posttraumatic stress disorder on 24-h urinary catecholamines and cortisol: results from the Mind Your Heart Study. Psychoneuroendocrinology 52, 83–91 (2015).
Kwok, M. K., Kawachi, I., Rehkopf, D. & Schooling, C. M. The role of cortisol in ischemic heart disease, ischemic stroke, type 2 diabetes, and cardiovascular disease risk factors: a bi-directional Mendelian randomization study. BMC Med. 18, 363 (2020).
Pan, X., Wang, Z., Wu, X., Wen, S. W. & Liu, A. Salivary cortisol in post-traumatic stress disorder: a systematic review and meta-analysis. BMC Psychiatry 18, 324 (2018).
Meewisse, M. L., Reitsma, J. B., de Vries, G. J., Gersons, B. P. & Olff, M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br. J. Psychiatry 191, 387–392 (2007).
Stalder, T. et al. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology 77, 261–274 (2017).
Oster, H. et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr. Rev. 38, 3–45 (2017).
Chrousos, G. P. Stress, chronic inflammation, and emotional and physical well-being: concurrent effects and chronic sequelae. J. Allergy Clin. Immunol. 106, S275–S291 (2000).
Ramamoorthy, S. & Cidlowski, J. A. Corticosteroids: mechanisms of action in health and disease. Rheum. Dis. Clin. North Am. 42, 15–31 (2016).
Bartolomucci, A. et al. The extended granin family: structure, function, and biomedical implications. Endocr. Rev. 32, 755–797 (2011).
Hirsch, D. & Zukowska, Z. NPY and stress 30 years later: the peripheral view. Cell Mol. Neurobiol. 32, 645–659 (2012).
Possenti, R. et al. Characterization of a novel peripheral pro-lipolytic mechanism in mice: role of VGF-derived peptide TLQP-21. Biochem. J. 441, 511–522 (2012).
Berger, J. M. et al. Mediation of the acute stress response by the skeleton. Cell Metab. 30, 890–902 (2019). This paper describes the role of osteoblasts and osteocalcin in the body’s metabolic regulation and modulation of the acute stress response and parasympathetic tone.
Rentscher, K. E. et al. Chronic stress exposure and daily stress appraisals relate to biological aging marker p16(INK4a). Psychoneuroendocrinology 102, 139–148 (2019).
Belsky, D. W. et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. eLife 9, 54870 (2020).
Noren Hooten, N., Pacheco, N. L., Smith, J. T. & Evans, M. K. The accelerated aging phenotype: the role of race and social determinants of health on aging. Ageing Res. Rev. 73, 101536 (2022).
Snyder-Mackler, N. et al. Social status alters immune regulation and response to infection in macaques. Science 354, 1041–1045 (2016). By manipulating the social status of individual macaques this animal study examined how stress affects immune function, demonstrating that social status influences the immune system at multiple levels, from immune cell numbers to gene expression and signalling pathways.
de Kloet, E. R., Meijer, O. C., de Nicola, A. F., de Rijk, R. H. & Joels, M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front. Neuroendocrinol. 49, 124–145 (2018).
Sapolsky, R. M., Romero, L. M. & Munck, A. U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 (2000).
Ogawa, A. et al. Roles of insulin resistance and beta-cell dysfunction in dexamethasone-induced diabetes. J. Clin. Invest. 90, 497–504 (1992).
Wiesner, T. D., Bluher, M., Windgassen, M. & Paschke, R. Improvement of insulin sensitivity after adrenalectomy in patients with pheochromocytoma. J. Clin. Endocrinol. Metab. 88, 3632–3636 (2003).
Utzschneider, K. M. & Kahn, S. E. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 91, 4753–4761 (2006).
Kivimaki, M. et al. Neighbourhood socioeconomic disadvantage, risk factors, and diabetes: a cohort study from childhood to middle age. Lancet Public Health 3, e365–e373 (2018).
Surwit, R. S. et al. Stress management improves long-term glycemic control in type 2 diabetes. Diabetes Care 25, 30–34 (2002).
Takahashi, A., Flanigan, M. E., McEwen, B. S. & Russo, S. J. Aggression, social stress, and the immune system in humans and animal models. Front. Behav. Neurosci. 12, 56 (2018).
Cohen, S. et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. U. S. A. 109, 5995–5999 (2012). The paper describes two viral challenge studies among people with and without stress, and provides support for the concept that prolonged exposure to a stressor can result in glucocorticoid receptor resistance and dysregulation of immune function, potentially contributing to the onset and progression of a wide range of diseases.
Winning, A., Glymour, M. M., McCormick, M. C., Gilsanz, P. & Kubzansky, L. D. Psychological distress across the life course and cardiometabolic risk: findings from the 1958 British Birth Cohort study. J. Am. Coll. Cardiol. 66, 1577–1586 (2015). This longitudinal analysis of the of the 1958 British Birth Cohort Study showed that psychological distress at any point in the life course is associated with higher cardiometabolic risk, the highest risk being evident among those with distress in both childhood and adulthood.
Deighton, S., Neville, A., Pusch, D. & Dobson, K. Biomarkers of adverse childhood experiences: a scoping review. Psychiatry Res. 269, 719–732 (2018).
Crick, D. C. P. et al. Associations between adverse childhood experiences and the novel inflammatory marker glycoprotein acetyls in two generations of the Avon Longitudinal Study of Parents and Children Birth Cohort. Brain Behav. Immun. 100, 112–120 (2022).
Berger, E. et al. Multi-cohort study identifies social determinants of systemic inflammation over the life course. Nat. Commun. 10, 773 (2019).
Danese, A. & McEwen, B. S. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav. 106, 29–39 (2012).
Ribeiro, A. I. et al. Neighbourhood socioeconomic deprivation and allostatic load: a multi-cohort study. Sci. Rep. 9, 8790 (2019).
Pivonello, R. et al. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol. 4, 611–629 (2016).
Hasenmajer, V. et al. The immune system in Cushing’s syndrome. Trends Endocrinol. Metab. 31, 655–669 (2020).
Passos, I. C. et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2, 1002–1012 (2015).
Olff, M. & van Zuiden, M. Neuroendocrine and neuroimmune markers in PTSD: pre-, peri- and post-trauma glucocorticoid and inflammatory dysregulation. Curr. Opin. Psychol. 14, 132–137 (2017).
Sumner, J. A. et al. Post-traumatic stress disorder symptoms and risk of hypertension over 22 years in a large cohort of younger and middle-aged women. Psychol. Med. 46, 3105–3116 (2016).
Shalev, A., Liberzon, I. & Marmar, C. Post-traumatic stress disorder. N. Engl. J. Med. 376, 2459–2469 (2017).
Zelinka, T. et al. Elevated inflammation markers in pheochromocytoma compared to other forms of hypertension. Neuroimmunomodulation 14, 57–64 (2007).
Liu, Z. et al. Associations of genetics, behaviors, and life course circumstances with a novel aging and healthspan measure: evidence from the Health and Retirement Study. PLoS Med. 16, e1002827 (2019).
Barzilai, N., Huffman, D. M., Muzumdar, R. H. & Bartke, A. The critical role of metabolic pathways in aging. Diabetes 61, 1315–1322 (2012).
Alpert, A. et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 25, 487–495 (2019).
Ferrucci, L. & Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018).
Crimmins, E. M. Social hallmarks of aging: suggestions for geroscience research. Ageing Res. Rev. 63, 101136 (2020).
Epel, E. S. The geroscience agenda: toxic stress, hormetic stress, and the rate of aging. Ageing Res. Rev. 63, 101167 (2020).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Childs, B. G., Durik, M., Baker, D. J. & van Deursen, J. M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 21, 1424–1435 (2015).
Lin, J. & Epel, E. S. Stress and telomere shortening: insights from cellular mechanisms. Ageing Res. Rev. 73, 101507 (2022).
Colich, N. L., Rosen, M. L., Williams, E. S. & McLaughlin, K. A. Biological aging in childhood and adolescence following experiences of threat and deprivation: a systematic review and meta-analysis. Psychol. Bull. 146, 721–764 (2020). A review and meta-analysis of 54 studies and over 116,010 participants synthesizing the evidence on the associations of early life adversity with pubertal timing and cellular ageing indicated by telomere length and DNA methylation age.
Epel, E. S. et al. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 101, 17312–17315 (2004).
Wolf, E. J. et al. Traumatic stress and accelerated DNA methylation age: a meta-analysis. Psychoneuroendocrinology 92, 123–134 (2018).
Reuben, A. et al. Association of neighborhood disadvantage in childhood with DNA methylation in young adulthood. JAMA Netw. Open 3, e206095 (2020).
Raffington, L. & Belsky, D. W. Integrating DNA methylation measures of biological aging into social determinants of health research. Curr. Environ. Health Rep. 9, 196–210 (2022).
Freni-Sterrantino, A. et al. Work-related stress and well-being in association with epigenetic age acceleration: a Northern Finland Birth Cohort 1966 Study. Aging 14, 1128–1156 (2022).
Turecki, G. & Meaney, M. J. Effects of the social environment and stress on glucocorticoid receptor gene methylation: a systematic review. Biol. Psychiatry 79, 87–96 (2016).
Zheng, Y., Ley, S. H. & Hu, F. B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 14, 88–98 (2018).
Carlsson, L. M. et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N. Engl. J. Med. 367, 695–704 (2012).
Pontzer, H. et al. Daily energy expenditure through the human life course. Science 373, 808–812 (2021).
Stefanaki, C., Pervanidou, P., Boschiero, D. & Chrousos, G. P. Chronic stress and body composition disorders: implications for health and disease. Hormones 17, 33–43 (2018).
Santosa, A. et al. Psychosocial risk factors and cardiovascular disease and death in a population-based cohort from 21 low-, middle-, and high-income countries. JAMA Netw. Open 4, e2138920 (2021).
Rautava, S. et al. Neighborhood socioeconomic disadvantage and childhood body mass index trajectories from birth to 7 years of age. Epidemiol 33, 121–130 (2022).
Ochoa, L. B. et al. Association of neighbourhood socioeconomic trajectories with preterm birth and small-for-gestational-age in the Netherlands: a nationwide population-based study. Lancet Reg. Health Eur. 10, 100205 (2021).
Razzoli, M. & Bartolomucci, A. The dichotomous effect of chronic stress on obesity. Trends Endocrinol. Metab. 27, 504–515 (2016).
Rosengren, A. et al. Psychosocial factors and obesity in 17 high-, middle- and low-income countries: the Prospective Urban Rural Epidemiologic (PURE) study. Int. J. Obes. 39, 1217–1223 (2015).
Oliver, G. & Wardle, J. Perceived effects of stress on food choice. Physiol. Behav. 66, 511–515 (1999).
Kivimäki, M. et al. Work stress, weight gain and weight loss: evidence for bidirectional effects of job strain on body mass index in the Whitehall II study. Int. J. Obes. 30, 982–987 (2006).
Nyberg, S. T. et al. Job strain in relation to body mass index: pooled analysis of 160 000 adults from 13 cohort studies. J. Int. Med. 272, 65–73 (2012).
Razzoli, M., Pearson, C., Crow, S. & Bartolomucci, A. Stress, overeating, and obesity: insights from human studies and preclinical models. Neurosci. Biobehav. Rev. 76, 154–162 (2017).
Virtanen, M. et al. Long working hours and alcohol use: systematic review and meta-analysis of published studies and unpublished individual participant data. BMJ 350, g7772 (2015).
Magnusson-Hanson, L. et al. Work stress, anthropometry, lung function, blood pressure, and blood-based biomarkers: a cross-sectional study of 43,593 French men and women. Sci. Rep. 7, 9282 (2017).
Fransson, E. I. et al. Job strain as a risk factor for leisure-time physical inactivity: an individual-participant meta-analysis of up to 170 000 men and women – The IPD-Work Consortium. Am. J. Epidemiol. 176, 1078–1089 (2012).
van den Berk-Clark, C. et al. Association between posttraumatic stress disorder and lack of exercise, poor diet, obesity, and co-occuring smoking: a systematic review and meta-analysis. Health Psychol. 37, 407–416 (2018).
Zhang, Y. et al. Sleep in posttraumatic stress disorder: a systematic review and meta-analysis of polysomnographic findings. Sleep. Med. Rev. 48, 101210 (2019).
Kim, E. J. & Dimsdale, J. E. The effect of psychosocial stress on sleep: a review of polysomnographic evidence. Behav. Sleep. Med. 5, 256–278 (2007).
Kalmbach, D. A., Anderson, J. R. & Drake, C. L. The impact of stress on sleep: pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders. J. Sleep. Res. 27, e12710 (2018).
Geiker, N. R. W. et al. Does stress influence sleep patterns, food intake, weight gain, abdominal obesity and weight loss interventions and vice versa? Obes. Rev. 19, 81–97 (2018).
Meier-Ewert, H. K. et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J. Am. Coll. Cardiol. 43, 678–683 (2004).
Kumari, M., Shipley, M., Stafford, M. & Kivimaki, M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. J. Clin. Endocrinol. Metab. 96, 1478–1485 (2011).
Hackett, R. A., Kivimaki, M., Kumari, M. & Steptoe, A. Diurnal cortisol patterns, future diabetes, and impaired glucose metabolism in the Whitehall II Cohort Study. J. Clin. Endocrinol. Metab. 101, 619–625 (2016).
Briancon-Marjollet, A. et al. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol. Metab. Syndr. 7, 25 (2015).
Iyegha, I. D., Chieh, A. Y., Bryant, B. M. & Li, L. Associations between poor sleep and glucose intolerance in prediabetes. Psychoneuroendocrinol 110, 104444 (2019).
Iob, E., Baldwin, J. R., Plomin, R. & Steptoe, A. Adverse childhood experiences, daytime salivary cortisol, and depressive symptoms in early adulthood: a longitudinal genetically informed twin study. Transl. Psychiatry 11, 420 (2021).
Lorant, V. et al. Socioeconomic inequalities in depression: a meta-analysis. Am. J. Epidemiol. 157, 98–112 (2003).
Richardson, R., Westley, T., Gariepy, G., Austin, N. & Nandi, A. Neighborhood socioeconomic conditions and depression: a systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 50, 1641–1656 (2015).
Madsen, I. E. H. et al. Job strain as a risk factor for clinical depression: systematic review and meta-analysis with additional individual participant data. Psychol. Med. 47, 1342–1356 (2017).
Tsirlin, A. et al. Pheochromocytoma: a review. Maturitas 77, 229–238 (2014).
Hashemian, F. et al. Anxiety, depression, and posttraumatic stress in Iranian survivors of chemical warfare. JAMA 296, 560–566 (2006).
Kawachi, I., Aida, J., Hikichi, H. & Kondo, K. Disaster resilience in aging populations: lessons from the 2011 Great East Japan earthquake and tsunami. J. R. Soc. N. Z. 50, 263–278 (2020).
Milaneschi, Y., Simmons, W. K., van Rossum, E. F. C. & Penninx, B. W. Depression and obesity: evidence of shared biological mechanisms. Mol. Psychiatry 24, 18–33 (2019).
Ulrich-Lai, Y. M. & Ryan, K. K. Neuroendocrine circuits governing energy balance and stress regulation: functional overlap and therapeutic implications. Cell Metab. 19, 910–925 (2014).
Momen, N. C. et al. Association between mental disorders and subsequent medical conditions. N. Engl. J. Med. 382, 1721–1731 (2020).
Tabak, A. G., Akbaraly, T. N., Batty, G. D. & Kivimaki, M. Depression and type 2 diabetes: a causal association? Lancet Diabetes Endocrinol. 2, 236–245 (2014).
Moulton, C. D., Pickup, J. C. & Ismail, K. The link between depression and diabetes: the search for shared mechanisms. Lancet Diabetes Endocrinol. 3, 461–471 (2015).
Lindekilde, N. et al. Prevalence of type 2 diabetes in psychiatric disorders: an umbrella review with meta-analysis of 245 observational studies from 32 systematic reviews. Diabetol 65, 440–456 (2022).
Soto-Angona, O. et al. Non-alcoholic fatty liver disease (NAFLD) as a neglected metabolic companion of psychiatric disorders: common pathways and future approaches. BMC Med. 18, 261 (2020).
Warrier, V. et al. Gene–environment correlations and causal effects of childhood maltreatment on physical and mental health: a genetically informed approach. Lancet Psychiat 8, 373–386 (2021).
Laitinen, T. T. et al. Childhood socioeconomic disadvantage and risk of fatty liver in adulthood: the Cardiovascular Risk in Young Finns Study. Hepatology 71, 67–75 (2019).
Rahimi, L., Rajpal, A. & Ismail-Beigi, F. Glucocorticoid-induced fatty liver disease. Diabetes Metab. Syndr. Obes. 13, 1133–1145 (2020).
Crowe, C. L. et al. Associations of loneliness and social isolation with health span and life span in the U.S. Health and Retirement Study. J. Gerontol. A Biol. Sci. Med. Sci. 76, 1997–2006 (2021).
Hughes, K. et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health 2, e356–366 (2017). A systematic review and meta-analysis highlighting the pervasive and strong association between adverse childhood experiences and a wide range of diseases throughout the life course with emphasis on the importance of addressing the various stressors that can occur in children’s lives.
Kivimaki, M. et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health 5, e140–e149 (2020). This outcome-wide study links socioeconomic adversity to increased risks of mental and behavioural disorders as well as a life-long cascade of diseases of the pancreas, liver, kidney, and vascular and respiratory systems and dementia.
Heikkila, K. et al. Job strain and COPD exacerbations: an individual-participant meta-analysis. Eur. Resp. J. 44, 247–251 (2014).
Heikkila, K. et al. Job strain and the risk of severe asthma exacerbations: a meta-analysis of individual-participant data from 100 000 European men and women. Allergy 69, 775–783 (2014).
Heikkila, K. et al. Work stress and risk of cancer: meta-analysis of 5700 incident cancer events in 116,000 European men and women. BMJ 346, f165 (2013).
Nyberg, S. T. et al. Job strain as a risk factor for type 2 diabetes: a pooled analysis of 124 808 men and women. Diabetes Care 37, 2268–2275 (2014). In this individual participant meta-analysis of 124,808 adults without diabetes mellitus from 13 European cohort studies, job strain was associated with a 1.15-fold increased risk of incident T2DM, with no evidence of differences in the association by sex.
Fransson, E. I. et al. Job strain and the risk of stroke: an individual-participant data meta-analysis. Stroke 46, 557–559 (2015).
Kivimaki, M. et al. Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet 380, 1491–1497 (2012).
Ervasti, J. et al. Long working hours and risk of 50 health conditions and mortality outcomes: a multicohort study in four European countries. Lancet Reg. Health Eur. 11, 100212 (2021).
Virtanen, M. et al. Long working hours and change in body weight: analysis of individual-participant data from 19 cohort studies. Int. J. Obes. 44, 1368–1375 (2020).
Liu, D. et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immun. 9, 30 (2013).
Daley-Yates, P. T. Inhaled corticosteroids: potency, dose equivalence and therapeutic index. Br. J. Clin. Pharmacol. 80, 372–380 (2015).
Sharma, S. T., Nieman, L. K. & Feelders, R. A. Comorbidities in Cushing’s disease. Pituitary 18, 188–194 (2015).
Dekkers, O. M. et al. Multisystem morbidity and mortality in Cushing’s syndrome: a cohort study. J. Clin. Endocrinol. Metab. 98, 2277–2284 (2013).
Pigeyre, M. et al. How obesity relates to socio-economic status: identification of eating behavior mediators. Int. J. Obes. 40, 1794–1801 (2016).
Agardh, E. E. et al. Burden of type 2 diabetes attributed to lower educational levels in Sweden. Popul. Health Metr. 9, 60 (2011).
Vancampfort, D. et al. Type 2 diabetes among people with posttraumatic stress disorder: systematic review and meta-analysis. Psychosom. Med. 78, 465–473 (2016).
Xu, T. et al. Onset of workplace bullying and risk of weight gain: a multicohort longitudinal study. Obesity 28, 2216–2223 (2020).
Xu, T. et al. Workplace bullying and violence as risk factors for type 2 diabetes: a multicohort study and meta-analysis. Diabetologia 61, 75–83 (2018).
Ul-Haq, Z., Mackay, D. F., Fenwick, E. & Pell, J. P. Meta-analysis of the association between body mass index and health-related quality of life among adults, assessed by the SF-36. Obesity 21, E322–E327 (2013).
Mommersteeg, P. M., Herr, R., Zijlstra, W. P., Schneider, S. & Pouwer, F. Higher levels of psychological distress are associated with a higher risk of incident diabetes during 18 year follow-up: results from the British Household Panel Survey. BMC Public Health 12, 1109 (2012).
Russ, T. C. et al. Association between psychological distress and liver disease mortality: a meta-analysis of individual study participants. Gastroenterol 148, 958–966 (2015).
Pena-Gralle, A. P. B. et al. Job strain and effort–reward imbalance as risk factors for type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Scand. J. Work. Environ. Health 48, 5–20 (2022).
Li, D. & Zou, Y. Causal effects of life course adiposity on chronic kidney disease: a Mendelian randomization study. Ann. Palliat. Med. 10, 10861–10869 (2021).
Cuevas, A. G. et al. Stressful life events and obesity in the United States: the role of nativity and length of residence. Am. J. Health Promot. 36, 190–193 (2022).
Wang, M. et al. Associations between stressful life events and diabetes: findings from the China Kadoorie Biobank study of 500,000 adults. J. Diabetes Investig. 10, 1215–1222 (2019).
Nordentoft, M. et al. Effort–reward imbalance at work and weight changes in a nationwide cohort of workers in Denmark. Am. J. Ind. Med. 63, 634–643 (2020).
Kouvonen, A., Kivimäki, M., Cox, S. J., Cox, T. & Vahtera, J. Relationship between work stress and body mass index among 45,810 female and male employees. Psychosom. Med. 67, 577–583 (2005).
Abdullah, A., Peeters, A., de Courten, M. & Stoelwinder, J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res. Clin. Pract. 89, 309–319 (2010).
Aune, D., Norat, T., Leitzmann, M., Tonstad, S. & Vatten, L. J. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 30, 529–542 (2015).
Zhao, J. et al. Triglyceride is an independent predictor of type 2 diabetes among middle-aged and older adults: a prospective study with 8-year follow-ups in two cohorts. J. Transl. Med. 17, 403 (2019).
Khera, R. et al. Cost-related medication nonadherence in adults with atherosclerotic cardiovascular disease in the United States, 2013 to 2017. Circulation 140, 2067–2075 (2019).
Steptoe, A. et al. Disruption of multisystem responses to stress in type 2 diabetes: investigating the dynamics of allostatic load. Proc. Natl. Acad. Sci. U. S. A. 111, 15693–15698 (2014).
Kivimaki, M. et al. Work stress and risk of death in men and women with and without cardiometabolic disease: a multicohort study. Lancet Diabetes Endocrinol. 6, 705–713 (2018).
Huang, W. et al. Psychological distress and all-cause, cardiovascular disease, cancer mortality among adults with and without diabetes. Clin. Epidemiol. 13, 555–565 (2021).
Dalsgaard, E. M. et al. Psychological distress, cardiovascular complications and mortality among people with screen-detected type 2 diabetes: follow-up of the ADDITION-Denmark trial. Diabetologia 57, 710–717 (2014).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020).
Fratiglioni, L., Marseglia, A. & Dekhtyar, S. Ageing without dementia: can stimulating psychosocial and lifestyle experiences make a difference? Lancet Neurol. 19, 533–543 (2020).
Tung, J., Archie, E. A., Altmann, J. & Alberts, S. C. Cumulative early life adversity predicts longevity in wild baboons. Nat. Commun. 7, 11181 (2016).
Bellis, M. A. et al. Measuring mortality and the burden of adult disease associated with adverse childhood experiences in England: a national survey. J. Public Health 37, 445–454 (2015).
Stringhini, S. et al. Socioeconomic status and the 25×25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1.7 million men and women. Lancet 389, 1229–1237 (2017).
Prior, A. et al. Bereavement, multimorbidity and mortality: a population-based study using bereavement as an indicator of mental stress. Psychol. Med. 48, 1437–1443 (2018).
Rutters, F. et al. The association between psychosocial stress and mortality is mediated by lifestyle and chronic diseases: the Hoorn Study. Soc. Sci. Med. 118, 166–172 (2014).
Falvey, J. R., Hajduk, A. M., Keys, C. R. & Chaudhry, S. I. Association of financial strain with mortality among older US adults recovering from an acute myocardial infarction. JAMA Intern. Med. 182, 445–448 (2022).
Prior, A. et al. The association between perceived stress and mortality among people with multimorbidity: a prospective population-based cohort study. Am. J. Epidemiol. 184, 199–210 (2016).
Batty, G. D., Hamer, M. & Gale, C. R. Life-course psychological distress and total mortality by middle age: the 1970 Birth Cohort Study. Epidemiology 32, 740–743 (2021).
Emerging Risk Factors Collaboration Association of cardiometabolic multimorbidity with mortality. JAMA 314, 52–60 (2015).
Singh-Manoux, A. et al. Clinical, socioeconomic, and behavioural factors at age 50 years and risk of cardiometabolic multimorbidity and mortality: a cohort study. PLoS Med. 15, e1002571 (2018). This cohort study with a 24-year follow-up examined transitions between health states and found that clinical risk factors (hypertension, obesity, high cholesterol and family history of diabetes mellitus or cardiovascular disease) are important predictors of first cardiometabolic disease, but socioeconomic disadvantage and unhealthy behaviours determine progression to multimorbidity.
Brunner, E. J. et al. Midlife contributors to socioeconomic differences in frailty during later life: a prospective cohort study. Lancet Public Health 3, e313–e322 (2018).
Courtin, E., Kim, S., Song, S., Yu, W. & Muennig, P. Can social policies improve health? A systematic review and meta-analysis of 38 randomized trials. Milbank Q. 98, 297–371 (2020).
Ludwig, J. et al. Neighborhoods, obesity, and diabetes – a randomized social experiment. N. Engl. J. Med. 365, 1509–1519 (2011). This real-life social experiment showed that moving from a socioeconomically disadvantaged neighbourhood to one with less socioeconomic disadvantage is associated with modest but potentially important reductions in the prevalence of severe obesity and T2DM.
Kivimaki, M. et al. Modifications to residential neighbourhood characteristics and risk of 79 common health conditions: a prospective cohort study. Lancet Public Health 6, e396–e407 (2021).
White, J. S. et al. Long-term effects of neighbourhood deprivation on diabetes risk: quasi-experimental evidence from a refugee dispersal policy in Sweden. Lancet Diabetes Endocrinol. 4, 517–524 (2016).
Chew, B. H., Vos, R. C., Metzendorf, M. I., Scholten, R. J. & Rutten, G. E. Psychological interventions for diabetes-related distress in adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 9, CD011469 (2017).
Winkley, K. et al. Psychological interventions to improve glycemic control in adults with type 2 diabetes: a systematic review and meta-analysis. BMJ Open. Diabetes Res. Care 8, 001150 (2020).
Crabb, D. W., Im, G. Y., Szabo, G., Mellinger, J. L. & Lucey, M. R. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 71, 306–333 (2020).
International Diabetes Federation. Global Guideline for Type 2 Diabetes. International Diabetes Federation https://www.idf.org/our-activities/advocacy-awareness/resources-and-tools/79:global-guideline-for-type-2-diabetes.html (2012).
International Diabetes Federation. Recommendations For Managing Type 2 Diabetes In Primary Care. International Diabetes Federation www.idf.org/managing-type2-diabetes (2017).
American Diabetes Association. 3. Prevention or delay of type 2 diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 44, S34–S39 (2021).
Chatterjee, S., Khunti, K. & Davies, M. J. Type 2 diabetes. Lancet 389, 2239–2251 (2017).
Young, C., Majolo, B., Heistermann, M., Schulke, O. & Ostner, J. Responses to social and environmental stress are attenuated by strong male bonds in wild macaques. Proc. Natl. Acad. Sci. U. S. A. 111, 18195–18200 (2014).
Fraser, O. N., Stahl, D. & Aureli, F. Stress reduction through consolation in chimpanzees. Proc. Natl. Acad. Sci. U. S. A. 105, 8557–8562 (2008).
Ruis, M. A. et al. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology 24, 285–300 (1999).
Carrillo-Alvarez, E., Kawachi, I. & Riera-Romani, J. Neighbourhood social capital and obesity: a systematic review of the literature. Obes. Rev. 20, 119–141 (2019).
Perez, E. et al. Neighbourhood community life and health: a systematic review of reviews. Health Place 61, 102238 (2020).
Acknowledgements
M.K. was supported by the UK Medical Research Council (S011676), Wellcome Trust, UK (221854/Z/20/Z), National Institute on Aging (NIH), US (R01AG056477), and the Academy of Finland (350426). A.B. was supported by NIH/NIDDK (DK117504, DK118150, DK102496), NIH/NHLBI (HL151740), NIH/NIA (AG043972) and MN Partnership for Biotechnology and Molecular Genomic #18.4. I.K. was supported by National Institute on Aging (R01 AG042463).
Author information
Authors and Affiliations
Contributions
All authors researched the literature for the article, provided substantial contributions to discussions of its content, wrote the article and undertook review and editing of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Theodore Robles and Stafford Lightman for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
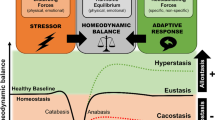
- Allostatic states
-
An allostatic state is a state in which there is a chronic deviation of regulatory systems away from their normal state of operation.
- Burnout
-
A syndrome resulting from excessive or prolonged stress and characterized by feelings of energy depletion or exhaustion, increased mental distancing, or feelings of negativism or cynicism related to one’s job and reduced professional efficacy.
- Ultradian
-
A rhythm with a period of recurrence shorter than 24 h.
- Polysomnography
-
A test protocol monitoring multiple physiological changes that occur during sleep (such as brain waves, eye and leg movement, heart rate and breathing) to study sleep and diagnose sleep disorders.
- Stroop colour–word interference task
-
A neuropsychological test to assess the ability to inhibit cognitive inference (a mismatch between the name of a colour and the colour it is printed on) used in the laboratory to induce stress in a study participant.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kivimäki, M., Bartolomucci, A. & Kawachi, I. The multiple roles of life stress in metabolic disorders. Nat Rev Endocrinol 19, 10–27 (2023). https://doi.org/10.1038/s41574-022-00746-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-022-00746-8
This article is cited by
-
A physicochemical-sensing electronic skin for stress response monitoring
Nature Electronics (2024)
-
Dynamic clustering via branched deep learning enhances personalization of stress prediction from mobile sensor data
Scientific Reports (2024)
-
Magnolol extends lifespan and improves age-related neurodegeneration in Caenorhabditis elegans via increase of stress resistance
Scientific Reports (2024)
-
Adipose-derived extracellular vesicles – a novel cross-talk mechanism in insulin resistance, non-alcoholic fatty liver disease, and polycystic ovary syndrome
Endocrine (2024)
-
Therapie des Typ-2-Diabetes
Die Diabetologie (2024)