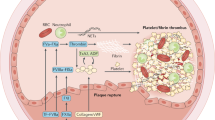
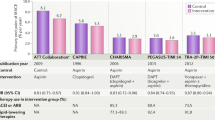
Abstract
Platelets have a crucial role in haemostasis and atherothrombosis. Pharmacological control of platelet hyper-reactivity has become a cornerstone in the prevention of thrombo-ischaemic complications in atherosclerotic diseases. Current antiplatelet therapies substantially improve clinical outcomes in patients with coronary artery disease, but at the cost of increased risk of bleeding. Beyond their role in thrombosis, platelets are known to regulate inflammatory (thrombo-inflammatory) and microcirculatory pathways. Therefore, controlling platelet hyper-reactivity might have implications for both tissue inflammation (myocardial ischaemia) and vascular inflammation (vulnerable plaque formation) to prevent atherosclerosis. In this Review, we summarize the pathophysiological role of platelets in acute myocardial ischaemia, vascular inflammation and atherosclerotic progression. Furthermore, we highlight current clinical concepts of antiplatelet therapy that have contributed to improving patient care and have facilitated more individualized therapy. Finally, we discuss novel therapeutic targets and compounds for antiplatelet therapy that are currently in preclinical development, some of which have a more favourable safety profile than currently approved drugs with regard to bleeding risk. These novel antiplatelet targets might offer new strategies to treat cardiovascular disease.
Key points
-
Antiplatelet therapy improves prognosis in patients with cardiovascular disease.
-
Bleeding is a major complication of antiplatelet therapy and is associated with an increased risk of death.
-
A personalized antithrombotic strategy that takes into account the individual’s risk of ischaemic and bleeding events has been developed.
-
Novel targets have been identified and new drugs developed to suppress platelet-dependent thrombosis with fewer effects on haemostasis than conventional antiplatelet therapies, thus minimizing the risk of bleeding complications.
-
Targeting novel intracellular molecules might improve the efficacy and safety of antiplatelet therapy by supporting the microcirculation and functional recovery after myocardial ischaemia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
No authors listed. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet 2, 349–360 (1988).
Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 373, 1849–1860 (2009).
Schomig, A. et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N. Engl. J. Med. 334, 1084–1089 (1996).
Urban, P. et al. Randomized evaluation of anticoagulation versus antiplatelet therapy after coronary stent implantation in high-risk patients: the Multicenter Aspirin and Ticlopidine Trial after Intracoronary Stenting (MATTIS). Circulation 98, 2126–2132 (1998).
Steinhubl, S. R. et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 288, 2411–2420 (2002).
Wiviott, S. D. et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 357, 2001–2015 (2007).
Wallentin, L. et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 361, 1045–1057 (2009).
Palmerini, T. et al. Bleeding-related deaths in relation to the duration of dual-antiplatelet therapy after coronary stenting. J. Am. Coll. Cardiol. 69, 2011–2022 (2017).
Gawaz, M., Langer, H. & May, A. E. Platelets in inflammation and atherogenesis. J. Clin. Invest. 115, 3378–3384 (2005).
Stark, K. & Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 18, 666–682 (2021).
Gawaz, M., Neumann, F. J., Ott, I., Schiessler, A. & Schomig, A. Platelet function in acute myocardial infarction treated with direct angioplasty. Circulation 93, 229–237 (1996).
Ott, I., Neumann, F. J., Gawaz, M., Schmitt, M. & Schomig, A. Increased neutrophil-platelet adhesion in patients with unstable angina. Circulation 94, 1239–1246 (1996).
Gawaz, M. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc. Res. 61, 498–511 (2004).
Weber, C. Platelets and chemokines in atherosclerosis: partners in crime. Circ. Res. 96, 612–616 (2005).
Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 368, 2004–2013 (2013).
Ziegler, M., Wang, X. & Peter, K. Platelets in cardiac ischaemia/reperfusion injury: a promising therapeutic target. Cardiovasc. Res. 115, 1178–1188 (2019).
Rohlfing, A. K. et al. ACKR3 regulates platelet activation and ischemia-reperfusion tissue injury. Nat. Commun. 13, 1823 (2022).
Kaplan, Z. S. & Jackson, S. P. The role of platelets in atherothrombosis. Hematol. Am. Soc. Hematol. Educ. Program. 2011, 51–61 (2011).
Davi, G. & Patrono, C. Platelet activation and atherothrombosis. N. Engl. J. Med. 357, 2482–2494 (2007).
Ruggeri, Z. M. Platelets in atherothrombosis. Nat. Med. 8, 1227–1234 (2002).
Nieswandt, B. et al. Glycoprotein VI but not α2β1 integrin is essential for platelet interaction with collagen. EMBO J. 20, 2120–2130 (2001).
Gawaz, M. & Vogel, S. Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood 122, 2550–2554 (2013).
Gawaz, M. P. et al. Ligand bridging mediates integrin alpha IIb beta 3 (platelet GPIIB-IIIA) dependent homotypic and heterotypic cell-cell interactions. J. Clin. Invest. 88, 1128–1134 (1991).
Massberg, S. et al. Platelets secrete stromal cell-derived factor 1α and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J. Exp. Med. 203, 1221–1233 (2006).
Henn, V. et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391, 591–594 (1998).
Massberg, S. et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J. Exp. Med. 196, 887–896 (2002).
Burger, P. C. & Wagner, D. D. Platelet P-selectin facilitates atherosclerotic lesion development. Blood 101, 2661–2666 (2003).
Gawaz, M. et al. Vitronectin receptor (αvβ3) mediates platelet adhesion to the luminal aspect of endothelial cells: implications for reperfusion in acute myocardial infarction. Circulation 96, 1809–1818 (1997).
Massberg, S. et al. A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo. J. Exp. Med. 197, 41–49 (2003).
Massberg, S. et al. Platelet adhesion via glycoprotein IIb integrin is critical for atheroprogression and focal cerebral ischemia: an in vivo study in mice lacking glycoprotein IIb. Circulation 112, 1180–1188 (2005).
Bultmann, A. et al. Impact of glycoprotein VI and platelet adhesion on atherosclerosis–a possible role of fibronectin. J. Mol. Cell Cardiol. 49, 532–542 (2010).
Seizer, P. et al. EMMPRIN (CD147) is a novel receptor for platelet GPVI and mediates platelet rolling via GPVI–EMMPRIN interaction. Thromb. Haemost. 101, 682–686 (2009).
Schonberger, T. et al. The dimeric platelet collagen receptor GPVI-Fc reduces platelet adhesion to activated endothelium and preserves myocardial function after transient ischemia in mice. Am. J. Physiol. Cell Physiol. 303, C757–C766 (2012).
Huo, Y. et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 9, 61–67 (2003).
Robbins, C. S. et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 19, 1166–1172 (2013).
Zeibig, S. et al. Effect of the oxLDL binding protein Fc-CD68 on plaque extension and vulnerability in atherosclerosis. Circ. Res. 108, 695–703 (2011).
Chatterjee, M. et al. Regulation of oxidized platelet lipidome: implications for coronary artery disease. Eur. Heart J. 38, 1993–2005 (2017).
Siegel-Axel, D., Daub, K., Seizer, P., Lindemann, S. & Gawaz, M. Platelet lipoprotein interplay: trigger of foam cell formation and driver of atherosclerosis. Cardiovasc. Res. 78, 8–17 (2008).
Harm, T. et al. Acute coronary syndrome is associated with a substantial change in the platelet lipidome. Cardiovasc. Res. 118, 1904–1916 (2022).
Chatterjee, M. et al. Platelet-derived CXCL12 regulates monocyte function, survival, differentiation into macrophages and foam cells through differential involvement of CXCR4-CXCR7. Cell Death Dis. 6, e1989 (2015).
Burgstahler, C. et al. Elevated coronary calcium scores are associated with higher residual platelet aggregation after clopidogrel treatment in patients with stable angina pectoris. Int. J. Cardiol. 135, 132–135 (2009).
Yun, K. H. et al. Relationship between platelet reactivity and culprit lesion morphology: an assessment from the ADAPT-DES intravascular ultrasound substudy. JACC Cardiovasc. Imaging 9, 849–854 (2016).
Matetzky, S. et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 109, 3171–3175 (2004).
Trip, M. D., Cats, V. M., van Capelle, F. J. & Vreeken, J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N. Engl. J. Med. 322, 1549–1554 (1990).
Geisler, T. et al. Early but not late stent thrombosis is influenced by residual platelet aggregation in patients undergoing coronary interventions. Eur. Heart J. 31, 59–66 (2010).
Muller, I. et al. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb. Haemost. 89, 783–787 (2003).
Cabeza, N. et al. Surface expression of collagen receptor Fc receptor-γ/glycoprotein VI is enhanced on platelets in type 2 diabetes and mediates release of CD40 ligand and activation of endothelial cells. Diabetes 53, 2117–2121 (2004).
Geisler, T. et al. Platelet response to clopidogrel is attenuated in diabetic patients undergoing coronary stent implantation. Diabetes Care 30, 372–374 (2007).
Dahlen, B. et al. The impact of platelet indices on clinical outcome in heart failure: results from the MyoVasc study. Esc. Heart Fail. 8, 2991–3001 (2021).
Valgimigli, M. et al. Duration of antiplatelet therapy after complex percutaneous coronary intervention in patients at high bleeding risk: a MASTER DAPT trial sub-analysis. Eur. Heart J. 43, 3100–3114 (2022).
Mauri, L. et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N. Engl. J. Med. 371, 2155–2166 (2014).
Bonaca, M. P. et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 372, 1791–1800 (2015).
Eikelboom, J. W. et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N. Engl. J. Med. 377, 1319–1330 (2017).
Xian, Y. et al. Association of discharge aspirin dose with outcomes after acute myocardial infarction: insights from the treatment with ADP receptor inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) study. Circulation 132, 174–181 (2015).
Mehta, S. R. et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet 376, 1233–1243 (2010).
Pettersen, A. A., Seljeflot, I., Abdelnoor, M. & Arnesen, H. High on-aspirin platelet reactivity and clinical outcome in patients with stable coronary artery disease: results from ASCET (Aspirin nonresponsiveness and Clopidogrel Endpoint Trial). J. Am. Heart Assoc. 1, e000703 (2012).
Chung, C. J. et al. Impact of high on-aspirin platelet reactivity on outcomes following successful percutaneous coronary intervention with drug-eluting stents. Am. Heart J. 205, 77–86 (2018).
Frelinger, A. L. 3rd et al. Residual arachidonic acid-induced platelet activation via an adenosine diphosphate-dependent but cyclooxygenase-1- and cyclooxygenase-2-independent pathway: a 700-patient study of aspirin resistance. Circulation 113, 2888–2896 (2006).
Chiang, N., Bermudez, E. A., Ridker, P. M., Hurwitz, S. & Serhan, C. N. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc. Natl Acad. Sci. USA 101, 15178–15183 (2004).
US Preventive Services Task Force. Aspirin use to prevent preeclampsia and related morbidity and mortality: US Preventive Services Task Force recommendation statement. JAMA 326, 1186–1191 (2021).
Reed, G. W. et al. Effect of aspirin coadministration on the safety of celecoxib, naproxen, or ibuprofen. J. Am. Coll. Cardiol. 71, 1741–1751 (2018).
Hohlfeld, T., Saxena, A. & Schror, K. High on treatment platelet reactivity against aspirin by non-steroidal anti-inflammatory drugs – pharmacological mechanisms and clinical relevance. Thromb. Haemost. 109, 825–833 (2013).
Cuisset, T. et al. Clinical implications of very low on-treatment platelet reactivity in patients treated with thienopyridine: the POBA study (predictor of bleedings with antiplatelet drugs). JACC Cardiovasc. Interv. 6, 854–863 (2013).
Campo, G. et al. Prospective evaluation of on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention relationship with gene polymorphisms and clinical outcome. J. Am. Coll. Cardiol. 57, 2474–2483 (2011).
Tantry, U. S. et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J. Am. Coll. Cardiol. 62, 2261–2273 (2013).
Bonello, L. et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 56, 919–933 (2010).
Geisler, T. et al. The residual platelet aggregation after deployment of intracoronary stent (PREDICT) score. J. Thromb. Haemost. 6, 54–61 (2008).
Nakamura, M. et al. Relationship between platelet reactivity and ischemic and bleeding events after percutaneous coronary intervention in East Asian patients: 1-year results of the PENDULUM registry. J. Am. Heart Assoc. 9, e015439 (2020).
Kim, H. K. et al. The East Asian paradox: an updated position statement on the challenges to the current antithrombotic strategy in patients with cardiovascular disease. Thromb. Haemost. 121, 422–432 (2021).
Brandt, J. T. et al. A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolite formation. Am. Heart J. 153, 66.e9–66.e16 (2007).
Alexopoulos, D. et al. Prasugrel overcomes high on-clopidogrel platelet reactivity post-stenting more effectively than high-dose (150-mg) clopidogrel: the importance of CYP2C19*2 genotyping. JACC Cardiovasc. Interv. 4, 403–410 (2011).
Wallentin, L. et al. Prasugrel achieves greater and faster P2Y12 receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur. Heart J. 29, 21–30 (2008).
Wang, Y. et al. Ticagrelor versus clopidogrel in CYP2C19 loss-of-function carriers with stroke or TIA. N. Engl. J. Med. 385, 2520–2530 (2021).
Cattaneo, M., Schulz, R. & Nylander, S. Adenosine-mediated effects of ticagrelor: evidence and potential clinical relevance. J. Am. Coll. Cardiol. 63, 2503–2509 (2014).
Nylander, S. et al. Ticagrelor inhibits human platelet aggregation via adenosine in addition to P2Y12 antagonism. J. Thromb. Haemost. 11, 1867–1876 (2013).
Huang, B. et al. Ticagrelor inhibits the NLRP3 inflammasome to protect against inflammatory disease independent of the P2Y12 signaling pathway. Cell Mol. Immunol. 18, 1278–1289 (2021).
Sexton, T. R. et al. Ticagrelor reduces thromboinflammatory markers in patients with pneumonia. JACC Basic Transl. Sci. 3, 435–449 (2018).
Morrow, D. A. et al. Vorapaxar in the secondary prevention of atherothrombotic events. N. Engl. J. Med. 366, 1404–1413 (2012).
Tricoci, P. et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N. Engl. J. Med. 366, 20–33 (2012).
Bonaca, M. P. et al. Acute limb ischemia and outcomes with vorapaxar in patients with peripheral artery disease: results from the trial to assess the effects of vorapaxar in preventing heart attack and stroke in patients with atherosclerosis-thrombolysis in myocardial infarction 50 (TRA2°P-TIMI 50). Circulation 133, 997–1005 (2016).
Bonaca, M. P. et al. Peripheral revascularization in patients with peripheral artery disease with vorapaxar: insights from the TRA 2°P-TIMI 50 trial. JACC Cardiovasc. Interv. 9, 2157–2164 (2016).
Cavender, M. A. et al. Vorapaxar in patients with diabetes mellitus and previous myocardial infarction: findings from the thrombin receptor antagonist in secondary prevention of atherothrombotic ischemic events-TIMI 50 trial. Circulation 131, 1047–1053 (2015).
Xu, H., Bonaca, M. P., Goodrich, E., Scirica, B. M. & Morrow, D. A. Efficacy and safety of vorapaxar for secondary prevention in low body weight in patients with atherosclerosis: analyses from the TRA 2°P-TIMI 50 trial. Eur. Heart J. Acute Cardiovasc. Care 10, 190–199 (2019).
Morrow, D. A. et al. Efficacy and safety of vorapaxar in patients with prior ischemic stroke. Stroke 44, 691–698 (2013).
Collet, J. P. et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 42, 1289–1367 (2021).
Gargiulo, G. et al. Cangrelor, tirofiban, and chewed or standard prasugrel regimens in patients with ST-segment-elevation myocardial infarction: primary results of the FABOLUS-FASTER trial. Circulation 142, 441–454 (2020).
Bledzka, K., Smyth, S. S. & Plow, E. F. Integrin αIIbβ3: from discovery to efficacious therapeutic target. Circ. Res. 112, 1189–1200 (2013).
Wu, F. et al. Efficacy and safety of a bridging strategy that uses intravenous platelet glycoprotein receptor inhibitors for patients undergoing surgery after coronary stent implantation: a meta-analysis. BMC Cardiovasc. Disord. 22, 125 (2022).
Merlini, P. A. et al. Thrombocytopenia caused by abciximab or tirofiban and its association with clinical outcome in patients undergoing coronary stenting. Circulation 109, 2203–2206 (2004).
The PURSUIT Trial Investigators. Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. N. Engl. J. Med. 339, 436–443 (1998).
The ESPRIT Investigators. Novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomised, placebo-controlled trial. Lancet 356, 2037–2044 (2000).
Valgimigli, M. et al. Tirofiban as adjunctive therapy for acute coronary syndromes and percutaneous coronary intervention: a meta-analysis of randomized trials. Eur. Heart J. 31, 35–49 (2010).
Siebler, M. et al. Safety of tirofiban in acute ischemic stroke: SaTIS trial. Stroke 42, 2388–2392 (2011).
Fu, Z., Xu, C., Liu, X., Wang, Z. & Gao, L. Safety and efficacy of tirofiban in acute ischemic stroke patients receiving endovascular treatment: a meta-analysis. Cerebrovasc. Dis. 49, 442–450 (2020).
Steg, P. G. et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet 382, 1981–1992 (2013).
Droppa, M. et al. Cangrelor in cardiogenic shock and after cardiopulmonary resuscitation: a global, multicenter, matched pair analysis with oral P2Y12 inhibition from the IABP-SHOCK II trial. Resuscitation 137, 205–212 (2019).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03551964 (2022).
Angiolillo, D. J. et al. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trial. JAMA 307, 265–274 (2012).
McNeil, J. J. et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N. Engl. J. Med. 379, 1509–1518 (2018).
ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N. Engl. J. Med. 379, 1529–1539 (2018).
Gaziano, J. M. et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 392, 1036–1046 (2018).
Yusuf, S. et al. Polypill with or without aspirin in persons without cardiovascular disease. N. Engl. J. Med. 384, 216–228 (2021).
Visseren, F. L. J. et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 42, 3227–3337 (2021).
Aggarwal, D. et al. P2Y12 inhibitor versus aspirin monotherapy for secondary prevention of cardiovascular events: meta-analysis of randomized trials. Eur. Heart J. Open 2, oeac019 (2022).
Schupke, S. et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N. Engl. J. Med. 381, 1524–1534 (2019).
Silvain, J. et al. Ticagrelor versus clopidogrel in elective percutaneous coronary intervention (ALPHEUS): a randomised, open-label, phase 3b trial. Lancet 396, 1737–1744 (2020).
Knuuti, J. et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 41, 407–477 (2020).
Montalescot, G. et al. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N. Engl. J. Med. 369, 999–1010 (2013).
Tarantini, G. et al. Timing of oral P2Y12 inhibitor administration in patients with non-ST-segment elevation acute coronary syndrome. J. Am. Coll. Cardiol. 76, 2450–2459 (2020).
Marquis-Gravel, G. et al. Post-discharge bleeding and mortality following acute coronary syndromes with or without PCI. J. Am. Coll. Cardiol. 76, 162–171 (2020).
Cao, D., Chandiramani, R., Chiarito, M., Claessen, B. E. & Mehran, R. Evolution of antithrombotic therapy in patients undergoing percutaneous coronary intervention: a 40-year journey. Eur. Heart J. 42, 339–351 (2021).
Costa, F. et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet 389, 1025–1034 (2017).
Urban, P. et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation 140, 240–261 (2019).
Sibbing, D. et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet 390, 1747–1757 (2017).
Cuisset, T. et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (Timing of Platelet Inhibition after Acute Coronary Syndrome) randomized study. Eur. Heart J. 38, 3070–3078 (2017).
Claassens, D. M. F. et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. N. Engl. J. Med. 381, 1621–1631 (2019).
Gimbel, M. et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet 395, 1374–1381 (2020).
Kim, H. S. et al. Durable polymer versus biodegradable polymer drug-eluting stents after percutaneous coronary intervention in patients with acute coronary syndrome: the Host-Reduce-Polytech-ACS Trial. Circulation 143, 1081–1091 (2021).
Kim, C. J. et al. Unguided de-escalation from ticagrelor to clopidogrel in stabilised patients with acute myocardial infarction undergoing percutaneous coronary intervention (TALOS-AMI): an investigator-initiated, open-label, multicentre, non-inferiority, randomised trial. Lancet 398, 1305–1316 (2021).
Kuno, T. et al. Comparison of unguided de-escalation versus guided selection of dual antiplatelet therapy after acute coronary syndrome: a systematic review and network meta-analysis. Circ. Cardiovasc. Interv. 15, e011990 (2022).
Galli, M. et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet 397, 1470–1483 (2021).
Vranckx, P. et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet 392, 940–949 (2018).
Mehran, R. et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N. Engl. J. Med. 381, 2032–2042 (2019).
Kim, B. K. et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA 323, 2407–2416 (2020).
Hong, S. J. et al. 1-Month dual-antiplatelet therapy followed by aspirin monotherapy after polymer-free drug-coated stent implantation: one-month DAPT trial. JACC Cardiovasc. Interv. 14, 1801–1811 (2021).
Kim, B. K. et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J. Am. Coll. Cardiol. 60, 1340–1348 (2012).
Feres, F. et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 310, 2510–2522 (2013).
Watanabe, H. et al. Comparison of clopidogrel monotherapy after 1 to 2 months of dual antiplatelet therapy with 12 months of dual antiplatelet therapy in patients with acute coronary syndrome: the STOPDAPT-2 ACS randomized clinical trial. JAMA Cardiol. 7, 407–417 (2022).
Bonaca, M. P. et al. Patient selection for long-term secondary prevention with ticagrelor: insights from PEGASUS-TIMI 54. Eur. Heart J. 43, 5037–5044 (2022).
Polzin, A. et al. Noncanonical effects of oral thrombin and factor Xa inhibitors in platelet activation and arterial thrombosis. Thromb. Haemost. 121, 122–130 (2021).
Petzold, T. et al. Rivaroxaban reduces arterial thrombosis by inhibition of FXa-driven platelet activation via protease activated receptor-1. Circ. Res. 126, 486–500 (2020).
Steffel, J. et al. The COMPASS trial: net clinical benefit of low-dose rivaroxaban plus aspirin as compared with aspirin in patients with chronic vascular disease. Circulation 142, 40–48 (2020).
Anand, S. S. et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 391, 219–229 (2018).
Bonaca, M. P. et al. Rivaroxaban in peripheral artery disease after revascularization. N. Engl. J. Med. 382, 1994–2004 (2020).
Versteeg, H. H., Heemskerk, J. W., Levi, M. & Reitsma, P. H. New fundamentals in hemostasis. Physiol. Rev. 93, 327–358 (2013).
Gurbel, P. A., Kuliopulos, A. & Tantry, U. S. G-protein-coupled receptors signaling pathways in new antiplatelet drug development. Arterioscler. Thromb. Vasc. Biol. 35, 500–512 (2015).
Rey, M. et al. The reversible P2Y12 antagonist ACT-246475 causes significantly less blood loss than ticagrelor at equivalent antithrombotic efficacy in rat. Pharmacol. Res. Perspect. 5, e00338 (2017).
Storey, R. F. et al. Pharmacodynamics, pharmacokinetics, and safety of single-dose subcutaneous administration of selatogrel, a novel P2Y12 receptor antagonist, in patients with chronic coronary syndromes. Eur. Heart J. 41, 3132–3140 (2020).
Sinnaeve, P. et al. Subcutaneous selatogrel inhibits platelet aggregation in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 75, 2588–2597 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04957719 (2023).
Coller, B. S. Platelet GPIIb/IIIa antagonists: the first anti-integrin receptor therapeutics. J. Clin. Invest. 100, S57–S60 (1997).
Coller, B. S. Blockade of platelet GPIIb/IIIa receptors as an antithrombotic strategy. Circulation 92, 2373–2380 (1995).
Ferguson, J. J., Waly, H. M. & Wilson, J. M. Fundamentals of coagulation and glycoprotein IIb/IIIa receptor inhibition. Eur. Heart J. 19, D3–D9 (1998).
Batchelor, W. B. et al. Randomized COMparison of platelet inhibition with abciximab, tiRofiban and eptifibatide during percutaneous coronary intervention in acute coronary syndromes: the COMPARE trial. Circulation 106, 1470–1476 (2002).
Li, J. et al. RUC-4: a novel αIIbβ3 antagonist for prehospital therapy of myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 34, 2321–2329 (2014).
Hohmann, J. D. et al. Delayed targeting of CD39 to activated platelet GPIIb/IIIa via a single-chain antibody: breaking the link between antithrombotic potency and bleeding? Blood 121, 3067–3075 (2013).
Nieswandt, B. & Watson, S. P. Platelet-collagen interaction: is GPVI the central receptor? Blood 102, 449–461 (2003).
Massberg, S. et al. Soluble glycoprotein VI dimer inhibits platelet adhesion and aggregation to the injured vessel wall in vivo. FASEB J. 18, 397–399 (2004).
Borst, O. & Gawaz, M. Glycoprotein VI – novel target in antiplatelet medication. Pharmacol. Ther. 217, 107630 (2021).
Ebrahim, M. et al. Dimeric glycoprotein VI binds to collagen but not to fibrin. Thromb. Haemost. 118, 351–361 (2018).
Ungerer, M. et al. Novel antiplatelet drug revacept (dimeric glycoprotein VI-Fc) specifically and efficiently inhibited collagen-induced platelet aggregation without affecting general hemostasis in humans. Circulation 123, 1891–1899 (2011).
Schupke, S. et al. Revacept, a novel inhibitor of platelet adhesion, in patients undergoing elective PCI – design and rationale of the randomized ISAR-PLASTER trial. Thromb. Haemost. 119, 1539–1545 (2019).
Mayer, K. et al. Efficacy and safety of revacept, a novel lesion-directed competitive antagonist to platelet glycoprotein VI, in patients undergoing elective percutaneous coronary intervention for stable ischemic heart disease: the randomized, double-blind, placebo-controlled ISAR-PLASTER phase 2 trial. JAMA Cardiol. 6, 753–761 (2021).
Uphaus, T. et al. Revacept, an inhibitor of platelet adhesion in symptomatic carotid stenosis: a multicenter randomized phase II trial. Stroke 53, 2718–2729 (2022).
Goebel, S. et al. The GPVI-Fc fusion protein revacept improves cerebral infarct volume and functional outcome in stroke. PLoS ONE 8, e66960 (2013).
Degen, H. et al. ADPase CD39 fused to glycoprotein VI-Fc boosts local antithrombotic effects at vascular lesions. J. Am. Heart Assoc. 6, e005991 (2017).
Nestele, J. A. et al. Characterization of GPVI- or GPVI-CD39-coated nanoparticles and their impact on in vitro thrombus formation. Int. J. Mol. Sci. 23, 11 (2021).
Baumer, Y. et al. The recombinant bifunctional protein αCD133-GPVI promotes repair of the infarcted myocardium in mice. J. Thromb. Haemost. 10, 1152–1164 (2012).
Lebozec, K., Jandrot-Perrus, M., Avenard, G., Favre-Bulle, O. & Billiald, P. Design, development and characterization of ACT017, a humanized Fab that blocks platelet’s glycoprotein VI function without causing bleeding risks. mAbs 9, 945–958 (2017).
Wichaiyo, S., Parichatikanond, W. & Rattanavipanon, W. Glenzocimab: a GPVI (glycoprotein VI)-targeted potential antiplatelet agent for the treatment of acute ischemic stroke. Stroke 53, 3506–3513 (2022).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT05070260 (2022).
Ruggeri, Z. M. The role of von Willebrand factor in thrombus formation. Thromb. Res. 120, S5–S9 (2007).
Reininger, A. J. et al. Mechanism of platelet adhesion to von Willebrand factor and microparticle formation under high shear stress. Blood 107, 3537–3545 (2006).
Gresele, P. & Momi, S. in Antiplatelet Agents (eds Gresele, P., Born, G., Patrono, C. & Page, C.) 287–309 (Springer, 2012).
De Meyer, S. F. et al. Binding of von Willebrand factor to collagen and glycoprotein Ibalpha, but not to glycoprotein IIb/IIIa, contributes to ischemic stroke in mice–brief report. Arterioscler. Thromb. Vasc. Biol. 30, 1949–1951 (2010).
Kleinschnitz, C. et al. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation 115, 2323–2330 (2007).
Tardif, J. C. et al. Effects of the P-selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention for non-ST-segment elevation myocardial infarction: results of the SELECT-ACS trial. J. Am. Coll. Cardiol. 61, 2048–2055 (2013).
Chatterjee, M., Rath, D. & Gawaz, M. Role of chemokine receptors CXCR4 and CXCR7 for platelet function. Biochem. Soc. Trans. 43, 720–726 (2015).
Witte, A. et al. The chemokine CXCL14 mediates platelet function and migration via direct interaction with CXCR4. Cardiovasc. Res. 117, 903–917 (2021).
Borst, O. et al. The inflammatory chemokine CXC motif ligand 16 triggers platelet activation and adhesion via CXC motif receptor 6-dependent phosphatidylinositide 3-kinase/Akt signaling. Circ. Res. 111, 1297–1307 (2012).
Rath, D. et al. Expression of stromal cell-derived factor-1 receptors CXCR4 and CXCR7 on circulating platelets of patients with acute coronary syndrome and association with left ventricular functional recovery. Eur. Heart J. 35, 386–394 (2014).
Chatterjee, M. et al. Macrophage migration inhibitory factor limits activation-induced apoptosis of platelets via CXCR7-dependent Akt signaling. Circ. Res. 115, 939–949 (2014).
Jackson, S. P. et al. PI3-kinase p110β: a new target for antithrombotic therapy. Nat. Med. 11, 507–514 (2005).
Cosemans, J. M. et al. Continuous signaling via PI3K isoforms β and γ is required for platelet ADP receptor function in dynamic thrombus stabilization. Blood 108, 3045–3052 (2006).
Gilio, K. et al. Non-redundant roles of phosphoinositide 3-kinase isoforms α and β in glycoprotein VI-induced platelet signaling and thrombus formation. J. Biol. Chem. 284, 33750–33762 (2009).
Gratacap, M. P. et al. Regulation and roles of PI3Kβ, a major actor in platelet signaling and functions. Adv. Enzym. Regul. 51, 106–116 (2011).
Canobbio, I. et al. Genetic evidence for a predominant role of PI3Kβ catalytic activity in ITAM- and integrin-mediated signaling in platelets. Blood 114, 2193–2196 (2009).
Laurent, P. A. et al. Platelet PI3Kβ and GSK3 regulate thrombus stability at a high shear rate. Blood 125, 881–888 (2015).
Martin, V. et al. Deletion of the p110β isoform of phosphoinositide 3-kinase in platelets reveals its central role in Akt activation and thrombus formation in vitro and in vivo. Blood 115, 2008–2013 (2010).
Zheng, Z. et al. Discovery and antiplatelet activity of a selective PI3Kβ inhibitor (MIPS-9922). Eur. J. Med. Chem. 122, 339–351 (2016).
Nylander, S. et al. Human target validation of phosphoinositide 3-kinase (PI3K)β: effects on platelets and insulin sensitivity, using AZD6482 a novel PI3Kβ inhibitor. J. Thromb. Haemost. 10, 2127–2136 (2012).
Nylander, S., Wagberg, F., Andersson, M., Skarby, T. & Gustafsson, D. Exploration of efficacy and bleeding with combined phosphoinositide 3-kinase β inhibition and aspirin in man. J. Thromb. Haemost. 13, 1494–1502 (2015).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT05363397 (2022).
Chen, X. et al. PDK1 regulates platelet activation and arterial thrombosis. Blood 121, 3718–3726 (2013).
Munzer, P. et al. PDK1 determines collagen-dependent platelet Ca2+ signaling and is critical to development of ischemic stroke in vivo. Arterioscler. Thromb. Vasc. Biol. 36, 1507–1516 (2016).
Geue, S. et al. Pivotal role of PDK1 in megakaryocyte cytoskeletal dynamics and polarization during platelet biogenesis. Blood 134, 1847–1858 (2019).
Dangelmaier, C. et al. PDK1 selectively phosphorylates Thr(308) on Akt and contributes to human platelet functional responses. Thromb. Haemost. 111, 508–517 (2014).
Borst, O. et al. The serum- and glucocorticoid-inducible kinase 1 (SGK1) influences platelet calcium signaling and function by regulation of Orai1 expression in megakaryocytes. Blood 119, 251–261 (2012).
Braun, A. et al. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood 113, 2056–2063 (2009).
Bergmeier, W. et al. R93W mutation in Orai1 causes impaired calcium influx in platelets. Blood 113, 675–678 (2009).
Lang, F. et al. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol. Rev. 86, 1151–1178 (2006).
Walker, B. et al. Impact of the serum- and glucocorticoid-inducible kinase 1 on platelet dense granule biogenesis and secretion. J. Thromb. Haemost. 13, 1325–1334 (2015).
Piazza, F. et al. Protein kinase CK2 in hematologic malignancies: reliance on a pivotal cell survival regulator by oncogenic signaling pathways. Leukemia 26, 1174–1179 (2012).
Nakanishi, K. et al. Phosphoinositide 3-kinase induced activation and cytoskeletal translocation of protein kinase CK2 in protease activated receptor 1-stimulated platelets. Thromb. Res. 126, 511–516 (2010).
Munzer, P. et al. CK2β regulates thrombopoiesis and Ca2+-triggered platelet activation in arterial thrombosis. Blood 130, 2774–2785 (2017).
Ryu, S. Y. & Kim, S. Evaluation of CK2 inhibitor (E)-3-(2,3,4,5-tetrabromophenyl)acrylic acid (TBCA) in regulation of platelet function. Eur. J. Pharmacol. 720, 391–400 (2013).
Ampofo, E. et al. Role of protein kinase CK2 in the dynamic interaction of platelets, leukocytes and endothelial cells during thrombus formation. Thromb. Res. 136, 996–1006 (2015).
Ezumi, Y., Shindoh, K., Tsuji, M. & Takayama, H. Physical and functional association of the Src family kinases Fyn and Lyn with the collagen receptor glycoprotein VI-Fc receptor γ chain complex on human platelets. J. Exp. Med. 188, 267–276 (1998).
Suzuki-Inoue, K. et al. Association of Fyn and Lyn with the proline-rich domain of glycoprotein VI regulates intracellular signaling. J. Biol. Chem. 277, 21561–21566 (2002).
Quek, L. S., Bolen, J. & Watson, S. P. A role for Bruton’s tyrosine kinase (Btk) in platelet activation by collagen. Curr. Biol. 8, 1137–1140 (1998).
Andre, P. et al. Critical role for Syk in responses to vascular injury. Blood 118, 5000–5010 (2011).
van Eeuwijk, J. M. et al. The novel oral Syk inhibitor, Bl1002494, protects mice from arterial thrombosis and thromboinflammatory brain infarction. Arterioscler. Thromb. Vasc. Biol. 36, 1247–1253 (2016).
Bussel, J. et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo-controlled trials. Am. J. Hematol. 93, 921–930 (2018).
Spalton, J. C. et al. The novel Syk inhibitor R406 reveals mechanistic differences in the initiation of GPVI and CLEC-2 signaling in platelets. J. Thromb. Haemost. 7, 1192–1199 (2009).
Harbi, M. H. et al. Antithrombotic effects of fostamatinib in combination with conventional antiplatelet drugs. Int. J. Mol. Sci. 23, 6982 (2022).
Busygina, K. et al. Oral bruton tyrosine kinase inhibitors selectively block atherosclerotic plaque-triggered thrombus formation in humans. Blood 131, 2605–2616 (2018).
Levade, M. et al. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood 124, 3991–3995 (2014).
Brown, J. R. et al. Incidence of and risk factors for major haemorrhage in patients treated with ibrutinib: an integrated analysis. Br. J. Haematol. 184, 558–569 (2019).
von Hundelshausen, P. & Siess, W. Bleeding by Bruton tyrosine kinase-inhibitors: dependency on drug type and disease. Cancers 13, 1103 (2021).
Smith, C. W. et al. The Btk inhibitor AB-95-LH34 potently inhibits atherosclerotic plaque-induced thrombus formation and platelet procoagulant activity. J. Thromb. Haemost. 20, 2939–2952 (2022).
Denzinger, V. et al. Optimizing platelet GPVI inhibition versus haemostatic impairment by the Btk inhibitors ibrutinib, acalabrutinib, ONO/GS-4059, BGB-3111 and evobrutinib. Thromb. Haemost. 119, 397–406 (2019).
Duan, R. et al. Effects of the Btk-inhibitors remibrutinib (LOU064) and rilzabrutinib (PRN1008) with varying Btk selectivity over tec on platelet aggregation and in vitro bleeding time. Front. Cardiovasc. Med. 8, 749022 (2021).
Manke, M. C. et al. ANXA7 regulates platelet lipid metabolism and Ca2+ release in arterial thrombosis. Circ. Res. 129, 494–507 (2021).
Yeung, J. et al. 12-lipoxygenase activity plays an important role in PAR4 and GPVI-mediated platelet reactivity. Thromb. Haemost. 110, 569–581 (2013).
Yeung, J. et al. Platelet 12-LOX is essential for FcγRIIa-mediated platelet activation. Blood 124, 2271–2279 (2014).
Peng, B. et al. Identification of key lipids critical for platelet activation by comprehensive analysis of the platelet lipidome. Blood 132, e1–e12 (2018).
Coffey, M. J. et al. Platelet 12-lipoxygenase activation via glycoprotein VI: involvement of multiple signaling pathways in agonist control of H(P)ETE synthesis. Circ. Res. 94, 1598–1605 (2004).
Adili, R. et al. First selective 12-LOX inhibitor, ML355, impairs thrombus formation and vessel occlusion in vivo with minimal effects on hemostasis. Arterioscler. Thromb. Vasc. Biol. 37, 1828–1839 (2017).
Li, H. et al. Targeting annexin A7 by a small molecule suppressed the activity of phosphatidylcholine-specific phospholipase C in vascular endothelial cells and inhibited atherosclerosis in apolipoprotein E−/− mice. Cell Death Dis. 4, e806 (2013).
Smolenski, A. Novel roles of cAMP/cGMP-dependent signaling in platelets. J. Thromb. Haemost. 10, 167–176 (2012).
Massberg, S. et al. Increased adhesion and aggregation of platelets lacking cyclic guanosine 3′,5′-monophosphate kinase I. J. Exp. Med. 189, 1255–1264 (1999).
Wen, L. et al. A shear-dependent NO-cGMP-cGKI cascade in platelets acts as an auto-regulatory brake of thrombosis. Nat. Commun. 9, 4301 (2018).
Sun, B. et al. Role of phosphodiesterase type 3A and 3B in regulating platelet and cardiac function using subtype-selective knockout mice. Cell Signal. 19, 1765–1771 (2007).
Gresele, P. & Momi, S. Novel approaches to antiplatelet therapy. Biochem. Pharmacol. 206, 115297 (2022).
Makhoul, S. et al. Effects of the NO/soluble guanylate cyclase/cGMP system on the functions of human platelets. Nitric Oxide 76, 71–80 (2018).
Toyoda, K. et al. Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high-risk ischaemic stroke in Japan: a multicentre, open-label, randomised controlled trial. Lancet Neurol. 18, 539–548 (2019).
Kim, B. J. et al. Cilostazol versus aspirin in ischemic stroke patients with high-risk cerebral hemorrhage: subgroup analysis of the PICASSO trial. Stroke 51, 931–937 (2020).
Gresele, P., Momi, S. & Guglielmini, G. Nitric oxide-enhancing or -releasing agents as antithrombotic drugs. Biochem. Pharmacol. 166, 300–312 (2019).
The ESPRIT Study Group. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet 367, 1665–1673 (2006).
Wallis, R. M., Corbin, J. D., Francis, S. H. & Ellis, P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am. J. Cardiol. 83 (Suppl. 1), 3–12 (1999).
Mullershausen, F. et al. Rapid nitric oxide-induced desensitization of the cGMP response is caused by increased activity of phosphodiesterase type 5 paralleled by phosphorylation of the enzyme. J. Cell Biol. 155, 271–278 (2001).
Wilson, L. S., Elbatarny, H. S., Crawley, S. W., Bennett, B. M. & Maurice, D. H. Compartmentation and compartment-specific regulation of PDE5 by protein kinase G allows selective cGMP-mediated regulation of platelet functions. Proc. Natl Acad. Sci. USA 105, 13650–13655 (2008).
Vanschoonbeek, K. et al. Initiating and potentiating role of platelets in tissue factor-induced thrombin generation in the presence of plasma: subject-dependent variation in thrombogram characteristics. J. Thromb. Haemost. 2, 476–484 (2004).
Reiss, C. et al. The sGC stimulator riociguat inhibits platelet function in washed platelets but not in whole blood. Br. J. Pharmacol. 172, 5199–5210 (2015).
Jordan, P. A. et al. A role for the thiol isomerase protein ERP5 in platelet function. Blood 105, 1500–1507 (2005).
Essex, D. W. & Li, M. Protein disulphide isomerase mediates platelet aggregation and secretion. Br. J. Haematol. 104, 448–454 (1999).
Lahav, J. et al. Sustained integrin ligation involves extracellular free sulfhydryls and enzymatically catalyzed disulfide exchange. Blood 100, 2472–2478 (2002).
Kim, K. et al. Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice. Blood 122, 1052–1061 (2013).
Cho, J., Furie, B. C., Coughlin, S. R. & Furie, B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J. Clin. Invest. 118, 1123–1131 (2008).
Jasuja, R. et al. Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J. Clin. Invest. 122, 2104–2113 (2012).
Lin, L. et al. Quercetin-3-rutinoside inhibits protein disulfide isomerase by binding to its b′x domain. J. Biol. Chem. 290, 23543–23552 (2015).
Stopa, J. D. et al. Protein disulfide isomerase inhibition blocks thrombin generation in humans by interfering with platelet factor V activation. JCI Insight 2, e89373 (2017).
Zwicker, J. I. et al. Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer. JCI Insight 4, 125851 (2019).
Chen, S. et al. Anti-thrombotic effects mediated by dihydromyricetin involve both platelet inhibition and endothelial protection. Pharmacol. Res. 167, 105540 (2021).
Flaumenhaft, R., Furie, B. & Zwicker, J. I. Therapeutic implications of protein disulfide isomerase inhibition in thrombotic disease. Arterioscler. Thromb. Vasc. Biol. 35, 16–23 (2015).
Tscharre, M., Michelson, A. D. & Gremmel, T. Novel antiplatelet agents in cardiovascular disease. J. Cardiovasc. Pharmacol. Ther. 25, 191–200 (2020).
Yang, W. et al. Discovery of 4-aryl-7-hydroxyindoline-based P2Y1 antagonists as novel antiplatelet agents. J. Med. Chem. 57, 6150–6164 (2014).
Bird, J. E. et al. A platelet target for venous thrombosis? P2Y1 deletion or antagonism protects mice from vena cava thrombosis. J. Thromb. Thrombolysis 34, 199–207 (2012).
Smolensky Koganov, E. et al. GLS-409, an antagonist of both P2Y1 and P2Y12, potently inhibits canine coronary artery thrombosis and reversibly inhibits human platelet activation. Sci. Rep. 8, 14529 (2018).
Delesque-Touchard, N. et al. SAR216471, an alternative to the use of currently available P2Y12 receptor inhibitors? Thromb. Res. 134, 693–703 (2014).
Bach, P. et al. Lead optimization of ethyl 6-aminonicotinate acyl sulfonamides as antagonists of the P2Y12 receptor. separation of the antithrombotic effect and bleeding for candidate drug AZD1283. J. Med. Chem. 56, 7015–7024 (2013).
Gurbel, P. A. et al. Cell-penetrating pepducin therapy targeting PAR1 in subjects with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 36, 189–197 (2016).
Wilson, S. J. et al. PAR4 (protease-activated receptor 4) antagonism with BMS-986120 inhibits human ex vivo thrombus formation. Arterioscler. Thromb. Vasc. Biol. 38, 448–456 (2018).
Meah, M. N. et al. Antithrombotic effects of combined PAR (protease-activated receptor)-4 antagonism and factor Xa inhibition. Arterioscler. Thromb. Vasc. Biol. 40, 2678–2685 (2020).
Lin, Y. C. et al. Selective inhibition of PAR4 (protease-activated receptor 4)-mediated platelet activation by a synthetic nonanticoagulant heparin analog. Arterioscler. Thromb. Vasc. Biol. 39, 694–703 (2019).
Billiald, P. et al. Targeting platelet GPVI with glenzocimab: a novel mechanism for inhibition. Blood Adv. https://doi.org/10.1182/bloodadvances.2022007863 (2022).
Voors-Pette, C. et al. Safety and tolerability, pharmacokinetics, and pharmacodynamics of ACT017, an antiplatelet GPVI (glycoprotein VI) Fab. Arterioscler. Thromb. Vasc. Biol. 39, 956–964 (2019).
Renaud, L. et al. Population pharmacokinetic/pharmacodynamic modeling of glenzocimab (ACT017) a glycoprotein VI inhibitor of collagen-induced platelet aggregation. J. Clin. Pharmacol. 60, 1198–1208 (2020).
Chang, C. H. et al. Trowaglerix venom polypeptides as a novel antithrombotic agent by targeting immunoglobulin-like domains of glycoprotein VI in platelet. Arterioscler. Thromb. Vasc. Biol. 37, 1307–1314 (2017).
Kageyama, S. et al. Pharmacokinetics and pharmacodynamics of AJW200, a humanized monoclonal antibody to von Willebrand factor, in monkeys. Arterioscler. Thromb. Vasc. Biol. 22, 187–192 (2002).
Wu, D. et al. Inhibition of the von Willebrand (VWF)-collagen interaction by an antihuman VWF monoclonal antibody results in abolition of in vivo arterial platelet thrombus formation in baboons. Blood 99, 3623–3628 (2002).
Gilbert, J. C. et al. First-in-human evaluation of anti von Willebrand factor therapeutic aptamer ARC1779 in healthy volunteers. Circulation 116, 2678–2686 (2007).
Scully, M. et al. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N. Engl. J. Med. 380, 335–346 (2019).
Knoebl, P. et al. Efficacy and safety of open-label caplacizumab in patients with exacerbations of acquired thrombotic thrombocytopenic purpura in the HERCULES study. J. Thromb. Haemost. 18, 479–484 (2020).
Peyvandi, F. et al. Caplacizumab prevents refractoriness and mortality in acquired thrombotic thrombocytopenic purpura: integrated analysis. Blood Adv. 5, 2137–2141 (2021).
Callewaert, F. et al. Evaluation of efficacy and safety of the anti-VWF Nanobody ALX-0681 in a preclinical baboon model of acquired thrombotic thrombocytopenic purpura. Blood 120, 3603–3610 (2012).
Wu, D., Meiring, M., Kotze, H. F., Deckmyn, H. & Cauwenberghs, N. Inhibition of platelet glycoprotein Ib, glycoprotein IIb/IIIa, or both by monoclonal antibodies prevents arterial thrombosis in baboons. Arterioscler. Thromb. Vasc. Biol. 22, 323–328 (2002).
Li, T. T. et al. A novel snake venom-derived GPIb antagonist, anfibatide, protects mice from acute experimental ischaemic stroke and reperfusion injury. Br. J. Pharmacol. 172, 3904–3916 (2015).
Nicolson, P. L. R. et al. Inhibition of Btk by Btk-specific concentrations of ibrutinib and acalabrutinib delays but does not block platelet aggregation mediated by glycoprotein VI. Haematologica 103, 2097–2108 (2018).
Goldmann, L. et al. Oral bruton tyrosine kinase inhibitors block activation of the platelet Fc receptor CD32a (FcγRIIA): a new option in HIT? Blood Adv. 3, 4021–4033 (2019).
Montalban, X. et al. Placebo-controlled trial of an oral BTK inhibitor in multiple sclerosis. N. Engl. J. Med. 380, 2406–2417 (2019).
Kamel, S. et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia 29, 783–787 (2015).
Gresele, P., Momi, S. & Falcinelli, E. Anti-platelet therapy: phosphodiesterase inhibitors. Br. J. Clin. Pharmacol. 72, 634–646 (2011).
Feijge, M. A., Ansink, K., Vanschoonbeek, K. & Heemskerk, J. W. Control of platelet activation by cyclic AMP turnover and cyclic nucleotide phosphodiesterase type-3. Biochem. Pharmacol. 67, 1559–1567 (2004).
Ghofrani, H. A., Simonneau, G. & Rubin, L. J. Riociguat for pulmonary hypertension. N. Engl. J. Med. 369, 2268 (2013).
Acknowledgements
The authors thank T. Castor (Eberhard Karls University of Tübingen, Germany) for help in the preparation of this article and M.-C. Manke (Eberhard Karls University of Tübingen, Germany) for help in preparing Fig. 4 for initial submission.
Author information
Authors and Affiliations
Contributions
M.G. discussed the content of the Review. All authors contributed to researching data for the article, and writing, reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.G. is the co-inventor of Revacept and is a co-founder of the biotechnology company AdvanceCor. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gawaz, M., Geisler, T. & Borst, O. Current concepts and novel targets for antiplatelet therapy. Nat Rev Cardiol 20, 583–599 (2023). https://doi.org/10.1038/s41569-023-00854-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-023-00854-6
This article is cited by
-
Machine learning insights into thrombo-ischemic risks and bleeding events through platelet lysophospholipids and acylcarnitine species
Scientific Reports (2024)
-
Large-scale lipidomics profiling reveals characteristic lipid signatures associated with an increased cardiovascular risk
Clinical Research in Cardiology (2023)
-
Critical shifts in lipid metabolism promote megakaryocyte differentiation and proplatelet formation
Nature Cardiovascular Research (2023)