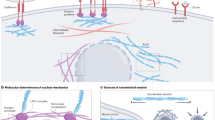
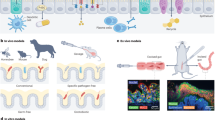
Abstract
The interactions among tumour cells, the tumour microenvironment (TME) and non-tumour tissues are of interest to many cancer researchers. Micro-engineering approaches and nanotechnologies are under extensive exploration for modelling these interactions and measuring them in situ and in vivo to investigate therapeutic vulnerabilities in cancer and extend a systemic view of tumour ecosystems. Here we highlight the greatest opportunities for improving the understanding of tumour ecosystems using microfluidic devices, bioprinting or organ-on-a-chip approaches. We also discuss the potential of nanosensors that can transmit information from within the TME or elsewhere in the body to address scientific and clinical questions about changes in chemical gradients, enzymatic activities, metabolic and immune profiles of the TME and circulating analytes. This Review aims to connect the cancer biology and engineering communities, presenting biomedical technologies that may expand the methodologies of the former, while inspiring the latter to develop approaches for interrogating cancer ecosystems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wu, F. et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat. Commun. 12, 2540 (2021).
Yan, Y. et al. Understanding heterogeneous tumor microenvironment in metastatic melanoma. PLoS ONE 14, e0216485 (2019).
Lee, Y. et al. XYZeq: spatially resolved single-cell RNA sequencing reveals expression heterogeneity in the tumor microenvironment. Sci. Adv. 7, eabg4755 (2021). Spatially resolved single-cell RNA-sequencing informs how cell type composition and cellular states relate to location within complex pathological tissue.
Elia, I. & Haigis, M. C. Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism. Nat. Metab. 3, 21–32 (2021).
Li, F. & Simon, M. C. Cancer cells don’t live alone: metabolic communication within tumor microenvironments. Dev. Cell 54, 183–195 (2020).
Lyssiotis, C. A. & Kimmelman, A. C. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 27, 863–875 (2017).
Belotti, D., Pinessi, D. & Taraboletti, G. Alternative vascularization mechanisms in tumor resistance to therapy. Cancers 13, 1912 (2021).
Klemm, F. et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell 181, 1643–1660.e17 (2020).
Rothschilds, A. M. & Wittrup, K. D. What, why, where, and when: bringing timing to immuno-oncology. Trends Immunol. 40, 12–21 (2019).
Kim, S. C., Clark, I. C., Shahi, P. & Abate, A. R. J. A. C. Single-cell RT–PCR in microfluidic droplets with integrated chemical lysis. Anal. Chem. 90, 1273–1279 (2018).
Chen Michelle, B. et al. Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proc. Natl Acad. Sci. USA 115, 7022–7027 (2018).
Ronaldson-Bouchard, K. et al. A multi-organ chip with matured tissue niches linked by vascular flow. Nat. Biomed. Eng. 6, 351–371 (2022).
Neufeld, L. et al. Microengineered perfusable 3D-bioprinted glioblastoma model for in vivo mimicry of tumor microenvironment. Sci. Adv. 7, eabi9119 (2021).
Kim, M. et al. Detection of ovarian cancer via the spectral fingerprinting of quantum-defect-modified carbon nanotubes in serum by machine learning. Nat. Biomed. Eng. 6, 267–275 (2022).
Gardner, L., Kostarelos, K., Mallick, P., Dive, C. & Hadjidemetriou, M. Nano-omics: nanotechnology-based multidimensional harvesting of the blood-circulating cancerome. Nat. Rev. Clin. Oncol. 19, 551–561 (2022).
Roberts, S. et al. Acid specific dark quencher QC1 pHLIP for multi-spectral optoacoustic diagnoses of breast cancer. Sci. Rep. 9, 8550 (2019).
Yamada, K. M. & Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell 130, 601–610 (2007).
Huang, L. et al. PDX-derived organoids model in vivo drug response and secrete biomarkers. JCI Insight 5, e135544 (2020).
Kim, J., Koo, B.-K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 21, 571–584 (2020).
Dao, V., Yuki, K., Lo, Y. H., Nakano, M. & Kuo, C. J. Immune organoids: from tumor modeling to precision oncology. Trends Cancer 8, 870–880 (2022).
Bose, S., Clevers, H. & Shen, X. Promises and challenges of organoid-guided precision medicine. Med 2, 1011–1026 (2021).
Neufeld, L., Yeini, E., Pozzi, S. & Satchi-Fainaro, R. 3D bioprinted cancer models: from basic biology to drug development. Nat. Rev. Cancer 22, 679–692 (2022).
Izumchenko, E. et al. Patient-derived xenografts effectively capture responses to oncology therapy in a heterogeneous cohort of patients with solid tumors. Ann. Oncol. 28, 2595–2605 (2017).
Yoshida, G. Applications of patient-derived tumor xenograft models and tumor organoids. J. Hematol. Oncol. 13, 4 (2020).
Katti, A., Diaz, B. J., Caragine, C. M., Sanjana, N. E. & Dow, L. E. CRISPR in cancer biology and therapy. Nat. Rev. Cancer 22, 259–279 (2022).
Liu, Y. et al. Mammalian models of chemically induced primary malignancies exploitable for imaging-based preclinical theragnostic research. Quant. Imaging Med. Surg. 5, 708 (2015).
Chen, S., Boda, S. K., Batra, S. K., Li, X. & Xie, J. Emerging roles of electrospun nanofibers in cancer research. Adv. Healthc. Mater. 7, 1701024 (2018).
Wang, Y. & Yao, Y. Nanofiber alignment mediates the pattern of single cell migration. Langmuir 36, 2129–2135 (2020).
Panagiotakopoulou, M. et al. A nanoprinted model of interstitial cancer migration reveals a link between cell deformability and proliferation. ACS Nano 10, 6437–6448 (2016).
Lemma, E. D. et al. Microenvironmental stiffness of 3D polymeric structures to study invasive rates of cancer cells. Adv. Healthc. Mater. 6, 1700888 (2017).
Yang, Y., Kulangara, K., Sia, J., Wang, L. & Leong, K. W. J. Engineering of a microfluidic cell culture platform embedded with nanoscale features. Lab Chip 11, 1638–1646 (2011).
Irimia, D., Charras, G., Agrawal, N., Mitchison, T. & Toner, M. Polar stimulation and constrained cell migration in microfluidic channels. Lab Chip 7, 1783–1790 (2007).
Pathak, A. & Kumar, S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl Acad. Sci. USA 109, 10334–10339 (2012).
Lin, B., Yin, T., Wu, Y. I., Inoue, T. & Levchenko, A. Interplay between chemotaxis and contact inhibition of locomotion determines exploratory cell migration. Nat. Commun. 6, 6619 (2015).
Wilson, K. et al. Mechanisms of leading edge protrusion in interstitial migration. Nat. Commun. 4, 2896 (2013).
Liu, Y. J. et al. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 160, 659–672 (2015).
Maiuri, P. et al. Actin flows mediate a universal coupling between cell speed and cell persistence. Cell 161, 374–386 (2015).
Lin, B. et al. Synthetic spatially graded Rac activation drives cell polarization and movement. Proc. Natl Acad. Sci. USA 109, E3668–E3677 (2012).
Fetah, K. L. et al. Cancer modeling‐on‐a‐chip with future artificial intelligence integration. Small 15, 1901985 (2019).
Han, B., Qu, C., Park, K., Konieczny, S. F. & Korc, M. Recapitulation of complex transport and action of drugs at the tumor microenvironment using tumor-microenvironment-on-chip. Cancer Lett. 380, 319–329 (2016).
Wu, Q. et al. Organ-on-a-chip: recent breakthroughs and future prospects. Biomed. Eng. Online 19, 9 (2020).
Zheng, F. et al. Organ‐on‐a‐chip systems: microengineering to biomimic living systems. Small 12, 2253–2282 (2016).
Orcheston-Findlay, L., Hashemi, A., Garrill, A. & Nock, V. A microfluidic gradient generator to simulate the oxygen microenvironment in cancer cell culture. Microelectron. Eng. 195, 107–113 (2018).
Ma, Y.-H. V., Middleton, K., You, L. & Sun, Y. J. M. A review of microfluidic approaches for investigating cancer extravasation during metastasis. Microsyst. Nanoeng. 4, 17104 (2018).
Chen, M. B. et al. On-chip human microvasculature assay for visualization and quantification of tumor cell extravasation dynamics. Nat. Protoc. 12, 865–880 (2017).
Rothbauer, M., Zirath, H. & Ertl, P. Recent advances in microfluidic technologies for cell-to-cell interaction studies. Lab Chip 18, 249–270 (2018).
Weinberg, F., Ramnath, N. & Nagrath, D. Reactive oxygen species in the tumor microenvironment: an overview. Cancers 11, 1191 (2019).
Place, T. L., Domann, F. E. & Case, A. J. Limitations of oxygen delivery to cells in culture: an underappreciated problem in basic and translational research. Free Radic. Biol. Med. 113, 311–322 (2017).
Ando, Y. et al. Evaluating CAR‐T cell therapy in a hypoxic 3D tumor model. Adv. Healthc. Mater. 8, 1900001 (2019).
Aung, A., Kumar, V., Theprungsirikul, J., Davey, S. K. & Varghese, S. An engineered tumor-on-a-chip device with breast cancer–immune cell interactions for assessing T-cell recruitment. Cancer Res. 80, 263–275 (2020).
Pavesi, A. et al. A 3D microfluidic model for preclinical evaluation of TCR-engineered T cells against solid tumors. JCI Insight 2, e89762 (2017). Three-dimensional modelling approach for systematic analysis of the hypoxic microenvironment.
Barmaki, S. et al. A microfluidic chip architecture enabling a hypoxic microenvironment and nitric oxide delivery in cell culture. Micromachines 11, 979 (2020).
Shirure, V. S. et al. Quantitative design strategies for fine control of oxygen in microfluidic systems. Lab Chip 20, 3036–3050 (2020).
Wang, X., Liu, Z. & Pang, Y. Concentration gradient generation methods based on microfluidic systems. RSC Adv. 7, 29966–29984 (2017).
Haessler, U., Kalinin, Y., Swartz, M. A. & Wu, M. An agarose-based microfluidic platform with a gradient buffer for 3D chemotaxis studies. Biomed. Microdevices 11, 827–835 (2009).
Allen, S. G. et al. Macrophages enhance migration in inflammatory breast cancer cells via RhoC GTPase signaling. Sci. Rep. 6, 39190 (2016).
Guo, Z. et al. M2 macrophages promote NSCLC metastasis by upregulating CRYAB. Cell Death Dis. 10, 1–11 (2019).
Ren, X., Alamri, A., Hipolito, J., Lin, F. & Kung, S. K. Applications of microfluidic devices in advancing NK-cell migration studies. Methods Enzymol. 631, 357–370 (2020).
Sai, J., Rogers, M., Hockemeyer, K., Wikswo, J. P. & Richmond, A. Study of chemotaxis and cell–cell interactions in cancer with microfluidic devices. Methods Enzymol. 570, 19–45 (2016).
Kwapiszewska, K., Michalczuk, A., Rybka, M., Kwapiszewski, R. & Brzózka, Z. A microfluidic-based platform for tumour spheroid culture, monitoring and drug screening. Lab Chip 14, 2096–2104 (2014).
Lee, S. W. L. et al. Integrated in silico and 3D in vitro model of macrophage migration in response to physical and chemical factors in the tumor microenvironment. Integr. Biol. 12, 90–108 (2020).
Hwang, H. et al. Human breast cancer-derived soluble factors facilitate CCL19-induced chemotaxis of human dendritic cells. Sci. Rep. 6, 30207 (2016).
Alexander, S., Koehl, G. E., Hirschberg, M., Geissler, E. K. & Friedl, P. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem. Cell Biol. 130, 1147–1154 (2008).
Wolf, K. & Friedl, P. Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol. 21, 736–744 (2011).
Bremer, C., Tung, C. H. & Weissleder, R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat. Med. 7, 743–748 (2001).
Fisher, K. E. et al. MT1-MMP- and Cdc42-dependent signaling co-regulate cell invasion and tunnel formation in 3D collagen matrices. J. Cell Sci. 122, 4558–4569 (2009).
Gaggioli, C. et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 9, 1392–1400 (2007). Modelling and optical imaging to monitor and control cancer cell migration in co-cultures of cancer cells and stromal fibroblasts.
Wolf, K. et al. Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 20, 931–941 (2009).
Park, D. et al. High-throughput microfluidic 3D cytotoxicity assay for cancer immunotherapy (CACI-IMPACT platform). Front. Immunol. 10, 1133 (2019).
Wirtz, D., Konstantopoulos, K. & Searson, P. C. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 11, 512–522 (2011).
Parlato, S. et al. 3D microfluidic model for evaluating immunotherapy efficacy by tracking dendritic cell behaviour toward tumor cells. Sci. Rep. 7, 1093 (2017).
Boussommier-Calleja, A. et al. The effects of monocytes on tumor cell extravasation in a 3D vascularized microfluidic model. Biomaterials 198, 180–193 (2019).
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Nguyen, M. et al. Dissecting effects of anti-cancer drugs and cancer-associated fibroblasts by on-chip reconstitution of immunocompetent tumor microenvironments. Cell Rep. 25, 3884–3893.e3 (2018). Micro-engineering approach to investigate intercellular interactions in the tumour microenvironment via single-cell tracking.
Yang, X. et al. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab Chip 18, 486–495 (2018).
Collins, T. et al. Spheroid-on-chip microfluidic technology for the evaluation of the impact of continuous flow on metastatic potential in cancer models in vitro. Biomicrofluidics 15, 044103 (2021).
Yi, H.-G. et al. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng. 3, 509–519 (2019). Bioprinting tumour models to reflect complexity and heterogeneity in the tumor microenvironment.
Panagiotakopoulou, M. et al. Cell cycle-dependent force transmission in cancer cells. Mol. Biol. Cell 29, 2528–2539 (2018).
Hansel, C. S. et al. Nanoneedle-mediated stimulation of cell mechanotransduction machinery. ACS Nano 13, 2913–2926 (2019).
Bergert, M. et al. Confocal reference free traction force microscopy. Nat. Commun. 7, 12814 (2016).
Zancla, A., Mozetic, P., Orsini, M., Forte, G. & Rainer, A. A primer to traction force microscopy. J. Biol. Chem. 298, 101867 (2022).
Schaaf, M. B., Garg, A. D. & Agostinis, P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 9, 115 (2018).
Ayuso, J. M. et al. Evaluating natural killer cell cytotoxicity against solid tumors using a microfluidic model. Oncoimmunology 8, 1553477 (2019).
Wimalachandra, D. C. et al. Microfluidic-based immunomodulation of immune cells using upconversion nanoparticles in simulated blood vessel–tumor system. ACS Appl. Mater. Interfaces 11, 37513–37523 (2019).
Sohn, L. L. et al. How can microfluidic and microfabrication approaches make experiments more physiologically relevant? Cell Syst. 11, 209–211 (2020).
Luque-González, M. A., Reis, R. L., Kundu, S. C. & Caballero, D. Human microcirculation-on-chip models in cancer research: key integration of lymphatic and blood vasculatures. Adv. Biosyst. 4, 2000045 (2020). Micro-engineering approach that incorporates lymphatic and blood vasculatures in a tumor-on-a-chip platform.
Szklanny, A. A. et al. 3D bioprinting of engineered tissue flaps with hierarchical vessel networks (VesselNet) for direct host-to-implant perfusion. Adv. Mater. 33, 2102661 (2021).
O’Connor, C., Brady, E., Zheng, Y., Moore, E. & Stevens, K. R. Engineering the multiscale complexity of vascular networks. Nat. Rev. Mater. 7, 702–716 (2022).
Bocci, F. et al. Toward understanding cancer stem cell heterogeneity in the tumor microenvironment. Proc. Natl Acad. Sci. USA 116, 148–157 (2019).
Williams, R. M. et al. Noninvasive ovarian cancer biomarker detection via an optical nanosensor implant. Sci. Adv. 4, eaaq1090 (2021).
Perillo, B. et al. ROS in cancer therapy: the bright side of the moon. Exp. Mol. Med. 52, 192–203 (2020).
Koch, C. J. Measurement of absolute oxygen levels in cells and tissues using oxygen sensors and 2-nitroimidazole EF5. Methods Enzymol. 352, 3–31 (2002).
Dranka, B. P. et al. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free. Radic. Biol. Med. 51, 1621–1635 (2011).
Smith, A. M., Mancini, M. C. & Nie, S. Second window for in vivo imaging. Nat. Nanotechnol. 4, 710–711 (2009).
Welsher, K., Sherlock, S. P. & Dai, H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc. Natl Acad. Sci. USA 108, 8943 (2011).
Mandal, A. K. et al. Fluorescent sp3 defect-tailored carbon nanotubes enable NIR-II single particle imaging in live brain slices at ultra-low excitation doses. Sci. Rep. 10, 5286 (2020).
Heller, D. A. et al. Peptide secondary structure modulates single-walled carbon nanotube fluorescence as a chaperone sensor for nitroaromatics. Proc. Natl Acad. Sci. USA 108, 8544–8549 (2011).
Heller, D. A. et al. Multimodal optical sensing and analyte specificity using single-walled carbon nanotubes. Nat. Nanotechnol. 4, 114–120 (2009).
Kim, J.-H. et al. The rational design of nitric oxide selectivity in single-walled carbon nanotube near-infrared fluorescence sensors for biological detection. Nat. Chem. 1, 473–481 (2009).
Zhang, J. et al. Single molecule detection of nitric oxide enabled by d(AT)15 DNA adsorbed to near infrared fluorescent single-walled carbon nanotubes. J. Am. Chem. Soc. 133, 567–581 (2011).
Jin, H. et al. Detection of single-molecule H2O2 signalling from epidermal growth factor receptor using fluorescent single-walled carbon nanotubes. Nat. Nanotechnol. 5, 302–309 (2010).
Yaari, Z. et al. Nanoreporter of an enzymatic suicide inactivation pathway. Nano Lett. 20, 7819–7827 (2020).
Zhao, M. et al. A tumor-microenvironment-responsive lanthanide–cyanine FRET sensor for NIR-II luminescence-lifetime in situ imaging of hepatocellular carcinoma. Adv. Mater. 32, 2001172 (2020).
Li, P. et al. Quantifying the fast dynamics of HClO in living cells by a fluorescence probe capable of responding to oxidation and reduction events within the time scale of milliseconds. Anal. Chem. 92, 12987–12995 (2020).
Zhao, Q. et al. Fluorescent/phosphorescent dual-emissive conjugated polymer dots for hypoxia bioimaging. Chem. Sci. 6, 1825–1831 (2015).
Jena, P. V. et al. A carbon nanotube optical reporter maps endolysosomal lipid flux. ACS Nano 11, 10689–10703 (2017).
Zhou, K. et al. Tunable, ultrasensitive pH-responsive nanoparticles targeting specific endocytic organelles in living cells. Angew. Chem. Int. Ed. 50, 6109–6114 (2011).
Ma, X. et al. Ultra-pH-sensitive nanoprobe library with broad pH tunability and fluorescence emissions. J. Am. Chem. Soc. 136, 11085–11092 (2014).
Ma, T. et al. Dual-ratiometric target-triggered fluorescent probe for simultaneous quantitative visualization of tumor microenvironment protease activity and pH in vivo. J. Am. Chem. Soc. 140, 211–218 (2018). Multiplexed nanosensor technology to detect protease activity and pH in the tumour microenvironment.
Hou, Y. et al. Protease-activated ratiometric fluorescent probe for pH mapping of malignant tumors. ACS Nano 9, 3199–3205 (2015).
Zhao, T. et al. A transistor-like pH nanoprobe for tumour detection and image-guided surgery. Nat. Biomed. Eng. 1, 0006 (2016).
Voskuil, F. J. et al. Exploiting metabolic acidosis in solid cancers using a tumor-agnostic pH-activatable nanoprobe for fluorescence-guided surgery. Nat. Commun. 11, 3257 (2020).
Andreev, O. A., Engelman, D. M. & Reshetnyak, Y. K. Targeting acidic diseased tissue: new technology based on use of the pH (low) insertion peptide (pHLIP). Chim. Oggi 27, 34–37 (2009).
Andreev, O. A. et al. pH (low) insertion peptide (pHLIP) inserts across a lipid bilayer as a helix and exits by a different path. Proc. Natl Acad. Sci. USA 107, 4081–4086 (2010).
Crawford, T. et al. pHLIP ICG for delineation of tumors and blood flow during fluorescence-guided surgery. Sci. Rep. 10, 18356 (2020). Nanosensor currently under clinical trial for image-guided surgery and other applications.
Bauer, D. et al. PET imaging of acidic tumor environment with 89Zr-labeled pHLIP probes. Front. Oncol. 12, 882541 (2022).
Kang, B., Austin, L. A. & El-Sayed, M. A. Observing real-time molecular event dynamics of apoptosis in living cancer cells using nuclear-targeted plasmonically enhanced Raman nanoprobes. ACS Nano 8, 4883–4892 (2014).
Li, S.-S. et al. Monitoring the changes of pH in lysosomes during autophagy and apoptosis by plasmon enhanced Raman imaging. Anal. Chem. 91, 8398–8405 (2019).
Guo, J. et al. Dynamic single-cell intracellular pH sensing using a SERS-active nanopipette. Analyst 145, 4852–4859 (2020).
Jamieson, L. E. et al. Targeted SERS nanosensors measure physicochemical gradients and free energy changes in live 3D tumor spheroids. Nanoscale 8, 16710–16718 (2016).
Chiappini, C. et al. Biodegradable nanoneedles for localized delivery of nanoparticles in vivo: exploring the biointerface. ACS Nano 9, 5500–5509 (2015).
Li, Z. & Zhang, H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol. Life Sci. 73, 377–392 (2016).
Leone Robert, D. et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 366, 1013–1021 (2019).
Lau, A. N. & Vander Heiden, M. G. Metabolism in the tumor microenvironment. Annu. Rev. Cancer Biol. 4, 17–40 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04473378 (2019).
Nascimento, R. A. S. et al. Single cell ‘glucose nanosensor’ verifies elevated glucose levels in individual cancer cells. Nano Lett. 16, 1194–1200 (2016).
Ma, Z. et al. In-situ monitoring of glucose metabolism in cancer cell microenvironments based on hollow fiber structure. Biosens. Bioelectron. 162, 112261 (2020).
Actis, P. et al. Compartmental genomics in living cells revealed by single-cell nanobiopsy. ACS Nano 8, 546–553 (2014).
Nashimoto, Y. et al. Evaluation of mRNA localization using double barrel scanning ion conductance microscopy. ACS Nano 10, 6915–6922 (2016).
Damaghi, M. et al. Chronic acidosis in the tumour microenvironment selects for overexpression of LAMP2 in the plasma membrane. Nat. Commun. 6, 8752 (2015).
Hui, S. et al. Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 (2017).
Brand, A. et al. DHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 24, 657–671 (2016).
Cali, J. J. et al. Compounds and methods for assaying redox state of metabolically active cells and methods for measuring NAD(P)/NAD(P)H. US patent US9273343B2 (2016).
Zheng, X. T., Yang, H. B. & Li, C. M. Optical detection of single cell lactate release for cancer metabolic analysis. Anal. Chem. 82, 5082–5087 (2010).
Jena, B. K. & Raj, C. R. Electrochemical biosensor based on integrated assembly of dehydrogenase enzymes and gold nanoparticles. Anal. Chem. 78, 6332–6339 (2006).
Xu, Y.-T. et al. A practical electrochemical nanotool for facile quantification of amino acids in single cell. Small 17, 2100503 (2021).
Snaebjornsson, M. T., Janaki-Raman, S. & Schulze, A. Greasing the wheels of the cancer machine: the role of lipid metabolism in cancer. Cell Metab. 31, 62–76 (2020).
Broadfield, L. A., Pane, A. A., Talebi, A., Swinnen, J. V. & Fendt, S.-M. Lipid metabolism in cancer: new perspectives and emerging mechanisms. Dev. Cell 56, 1363–1393 (2021).
Mancini, R. et al. Metabolic features of cancer stem cells: the emerging role of lipid metabolism. Oncogene 37, 2367–2378 (2018).
Hendrikx, T., Walenbergh, S. M. A., Hofker, M. H. & Shiri-Sverdlov, R. Lysosomal cholesterol accumulation: driver on the road to inflammation during atherosclerosis and non-alcoholic steatohepatitis. Obes. Rev. 15, 424–433 (2014).
Michelotti, G. A., Machado, M. V. & Diehl, A. M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 10, 656–665 (2013).
Mehlem, A., Hagberg, C. E., Muhl, L., Eriksson, U. & Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 8, 1149–1154 (2013).
Hofmann, K. et al. A novel alkyne cholesterol to trace cellular cholesterol metabolism and localization. J. Lipid Res. 55, 583–591 (2014).
Takatori, S., Mesman, R. & Fujimoto, T. Microscopic methods to observe the distribution of lipids in the cellular membrane. Biochemistry 53, 639–653 (2014).
Mondal, S., Rakshit, A., Pal, S. & Datta, A. Cell permeable ratiometric fluorescent sensors for imaging phosphoinositides. ACS Chem. Biol. 11, 1834–1843 (2016).
Galassi, T. V. et al. An optical nanoreporter of endolysosomal lipid accumulation reveals enduring effects of diet on hepatic macrophages in vivo. Sci. Transl. Med. 10, eaar2680 (2018).
Yong, C., Stewart, G. D. & Frezza, C. Oncometabolites in renal cancer. Nat. Rev. Nephrol. 16, 156–172 (2020).
Liu, Y. & Yang, C. Oncometabolites in cancer: current understanding and challenges. Cancer Res. 81, 2820–2823 (2021).
Yang, M., Soga, T. & Pollard, P. J. Oncometabolites: linking altered metabolism with cancer. J. Clin. Invest. 123, 3652–3658 (2013).
Kaper, T. et al. Nanosensor detection of an immunoregulatory tryptophan influx/kynurenine efflux cycle. PLoS Biol. 5, e257 (2007).
Fehr, M., Lalonde, S., Lager, I., Wolff, M. W. & Frommer, W. B. In vivo imaging of the dynamics of glucose uptake in the cytosol of COS-7 cells by fluorescent nanosensors. J. Biol. Chem. 278, 19127–19133 (2003).
Zhang, J. et al. Molecular recognition using corona phase complexes made of synthetic polymers adsorbed on carbon nanotubes. Nat. Nanotechnol. 8, 959–968 (2013). Strategy to develop optical nanosensors based on synthetic molecular recognition.
Amir, D., Hendler-Neumark, A., Wulf, V., Ehrlich, R. & Bisker, G. Oncometabolite fingerprinting using fluorescent single-walled carbon nanotubes. Adv. Mater. Interfaces 9, 2101591 (2022).
Ježek, P. 2-Hydroxyglutarate in cancer cells. Antioxid. Redox Signal. 33, 903–926 (2020).
Ehrlich, R., Hendler-Neumark, A., Wulf, V., Amir, D. & Bisker, G. Optical nanosensors for real-time feedback on insulin secretion by β-cells. Small 17, 2101660 (2021).
Levy, J. M. M., Towers, C. G. & Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 17, 528–542 (2017).
Linder, B. & Kögel, D. Autophagy in cancer cell death. Biology 8, 82 (2019).
Huefner, A. et al. Characterization and visualization of vesicles in the endo-lysosomal pathway with surface-enhanced Raman spectroscopy and chemometrics. ACS Nano 10, 307–316 (2016).
Chen, Z. et al. Single gold@silver nanoprobes for real-time tracing the entire autophagy process at single-cell level. J. Am. Chem. Soc. 137, 1903–1908 (2015).
Ou, Y.-C., Wen, X. & Bardhan, R. Cancer immunoimaging with smart nanoparticles. Trends Biotechnol. 38, 388–403 (2020).
Dauphin-Ducharme, P. et al. Electrochemical aptamer-based sensors for improved therapeutic drug monitoring and high-precision, feedback-controlled drug delivery. ACS Sens. 4, 2832–2837 (2019).
Arroyo-Currás, N. et al. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc. Natl Acad. Sci. USA 114, 645–650 (2017).
Parolo, C. et al. Real-time monitoring of a protein biomarker. ACS Sens. 5, 1877–1881 (2020).
Zheng, A. et al. CD16/PD-L1 bi-specific aptamer for cancer immunotherapy through recruiting NK cells and acting as immunocheckpoint blockade. Mol. Ther. Nucleic 27, 998–1009 (2022).
Cui, D., Li, J., Zhao, X., Pu, K. & Zhang, R. Semiconducting polymer nanoreporters for near-infrared chemiluminescence imaging of immunoactivation. Adv. Mater. 32, 1906314 (2020).
Dheer, D., Nicolas, J. & Shankar, R. Cathepsin-sensitive nanoscale drug delivery systems for cancer therapy and other diseases. Adv. Drug Deliv. Rev. 151–152, 130–151 (2019).
Shahriari, M. et al. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Rel. 308, 172–189 (2019).
Vizovisek, M., Ristanovic, D., Menghini, S., Christiansen, M. G. & Schuerle, S. The tumor proteolytic landscape: a challenging frontier in cancer diagnosis and therapy. Int. J. Mol. Sci. 22, 2514 (2021).
Bengsch, F. et al. Cell type-dependent pathogenic functions of overexpressed human cathepsin B in murine breast cancer progression. Oncogene 33, 4474–4484 (2014).
Scott, J. I., Deng, Q. & Vendrell, M. Near-infrared fluorescent probes for the detection of cancer-associated proteases. ACS Chem. Biol. 16, 1304–1317 (2021).
Kwon, E. J., Dudani, J. S. & Bhatia, S. N. Ultrasensitive tumour-penetrating nanosensors of protease activity. Nat. Biomed. Eng. 1, 0054 (2017). Development of optical nanosensors that are selectively activated by tumour-specific protease to detect small tumours.
Dudani, J. S., Jain, P. K., Kwong, G. A., Stevens, K. R. & Bhatia, S. N. Photoactivated spatiotemporally-responsive nanosensors of in vivo protease activity. ACS Nano 9, 11708–11717 (2015).
Myochin, T. et al. Development of a series of near-infrared dark quenchers based on Si-rhodamines and their application to fluorescent probes. J. Am. Chem. Soc. 137, 4759–4765 (2015).
Fernald, K. & Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 23, 620–633 (2013).
Liu, C. et al. A graphene oxide nanosensor enables the co-delivery of aptamer and peptide probes for fluorescence imaging of a cascade reaction in apoptotic signaling. Analyst 143, 208–214 (2018).
Zhang, X. et al. A fluorescent turn on nanoprobe for simultaneous visualization of dual-targets involved in cell apoptosis and drug screening in living cells. Nanoscale 9, 10861–10868 (2017).
Sindhwani, S. et al. The entry of nanoparticles into solid tumours. Nat. Mater. 19, 566–575 (2020).
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Christian, S. et al. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J. Cell Biol. 163, 871–878 (2003).
Safaee, M. M., Gravely, M. & Roxbury, D. A wearable optical microfibrous biomaterial with encapsulated nanosensors enables wireless monitoring of oxidative stress. Adv. Healthc. Mater. 31, 2006254 (2021).
Harvey, J. D. et al. A carbon nanotube reporter of microRNA hybridization events in vivo. Nat. Biomed. Eng. 1, 0041 (2017). Minimally invasive implantable sensor technology to longitudinally monitor tumour biomarkers in vivo.
Horowitz, L. et al. Multiplexed drug testing of tumor slices using a microfluidic platform. npj Precis. Oncol. 4, 12 (2020).
Wong, B. S. et al. A microfluidic cell-migration assay for the prediction of progression-free survival and recurrence time of patients with glioblastoma. Nat. Biomed. Eng. 5, 26–40 (2021).
Phillips, E. et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 6, 260ra149 (2014).
Serkova, N. J. Nanoparticle-based magnetic resonance imaging on tumor-associated macrophages and inflammation. Front. Immunol. 8, 590 (2017).
Guo, J. et al. Auto-affitech: an automated ligand binding affinity evaluation platform using digital microfluidics with a bidirectional magnetic separation method. Lab Chip 20, 1577–1585 (2020).
Song, J. W. et al. Microfluidic endothelium for studying the intravascular adhesion of metastatic breast cancer cells. PLoS ONE 4, e5756 (2009).
Eduati, F. et al. A microfluidics platform for combinatorial drug screening on cancer biopsies. Nat. Commun. 9, 2434 (2018).
Aref, A. R. et al. Screening therapeutic EMT blocking agents in a three-dimensional microenvironment. Integr. Biol. 5, 381–389 (2013).
Zhao, S.-P. et al. Three-dimensional cell culture and drug testing in a microfluidic sidewall-attached droplet array. Anal. Chem. 89, 10153–10157 (2017).
Shang, M. et al. Microfluidic studies of hydrostatic pressure-enhanced doxorubicin resistance in human breast cancer cells. Lab Chip 21, 746–754 (2021).
Hassell, B. A. et al. Human organ chip models recapitulate orthotopic lung cancer growth, therapeutic responses, and tumor dormancy in vitro. Cell Rep. 21, 508–516 (2017).
Ying, L. et al. Cancer associated fibroblast-derived hepatocyte growth factor inhibits the paclitaxel-induced apoptosis of lung cancer A549 cells by up-regulating the PI3K/Akt and GRP78 signaling on a microfluidic platform. PLoS ONE 10, e0129593 (2015).
Mann, F. A., Galonska, P., Herrmann, N., & Kruss, S. Quantum defects as versatile anchors for carbon nanotube functionalization. Nat. Protoc. 17, 727–747 (2022).
Kwon, H. et al. Optical probing of local pH and temperature in complex fluids with covalently functionalized, semiconducting carbon nanotubes. J. Phys. Chem. C 119, 3733–3739 (2015).
Zeng, W. et al. Ratiometric imaging of MMP-2 activity facilitates tumor detection using activatable near-infrared fluorescent semiconducting polymer nanoparticles. Small 17, 2101924 (2021).
Tavaré, R. et al. An effective immuno-PET imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res. 76, 73–82 (2016).
Dai, X., Zhou, W., Gao, T., Liu, J. & Lieber, C. M. Three-dimensional mapping and regulation of action potential propagation in nanoelectronics-innervated tissues. Nat. Nanotechnol. 11, 776–782 (2016).
Sontheimer-Phelps, A., Hassell, B. A. & Ingber, D. E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 19, 65–81 (2019).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04996355 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04755907 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT05130801 (2021).
Daldrup-Link, H. E. et al. MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin. Cancer Res. 17, 5695–5704 (2011).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04682847 (2020).
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic: an update post COVID-19 vaccines. Bioeng. Transl. Med. 6, e10246 (2021).
Benezra, M. et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 121, 2768–2780 (2011).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02106598 (2014).
Martin, K. H. & Dayton, P. A. Current status and prospects for microbubbles in ultrasound theranostics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 5, 329–345 (2013).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03199274 (2017).
Manzari, M. T. et al. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 6, 351–370 (2021).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Gurbatri, C. R., Arpaia, N. & Danino, T. Engineering bacteria as interactive cancer therapies. Science 378, 858–864 (2022).
Li, C. & Samulski, R. J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 21, 255–272 (2020).
Shipman, S. L., Nivala, J., Macklis, J. D. & Church, G. M. Molecular recordings by directed CRISPR spacer acquisition. Science 353, aaf1175 (2016).
Acknowledgements
This work was supported in part by the NIH (R01-CA215719, R01-NS116353, R01-NS122987, R01-DK129299 and the Cancer Center Support Grant, P30-CA008748), the National Science Foundation CAREER Award (1752506), the American Cancer Society Research Scholar Grant (GC230452), the Ara Parseghian Foundation, the Honorable Tina Brozman Foundation for Ovarian Cancer Research, the Ovarian Cancer Research Alliance (CRDGAI-2023-3-1003), the Pershing Square Sohn Cancer Research Alliance, the Expect Miracles Foundation — Financial Services Against Cancer, Emerson Collective, the Experimental Therapeutics Center, William H. Goodwin and Alice Goodwin and the Commonwealth Foundation for Cancer Research, Burroughs Wellcome Funds, AACR, Stand Up to Cancer. M.K. was supported by the NIH (K99-EB033580) and the Marie-Josée Kravis Women in Science Endeavor Postdoctoral Fellowship. M.P. was supported by an NIH grant (5T32CA062948) and the Tow Foundation Postdoctoral Fellowship. S.B.R. was supported by the MERIT Mandel Fellowship, Memorial Sloan Kettering Cancer Center.
Author information
Authors and Affiliations
Contributions
M.K., M.P., C.C. and S.B.R. researched data for the article. M.K., M.P., C.C., K.G., T.T. and D.A.H. contributed substantially to discussion of the content. M.K., M.P., C.C., S.B.R. and D.A.H wrote the article. M.K., K.G., T.T. and D.A.H reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.A.H. is a co-founder and officer with equity interest in Lime Therapeutics, Inc. and co-founder with equity interest in Selectin Therapeutics Inc. and Resident Diagnostics, Inc. and a member of the scientific advisory board of Concarlo Therapeutics, Inc., Nanorobotics Inc. and Mediphage Bioceuticals, Inc. T.T. has research support from ONO Pharma USA, Inc. (unrelated to this work) and is a member of the scientific advisory board of Lime Therapeutics, Inc. with equity interest. M.K., M.P., C.C., S.B.R. and K.G. declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Xiaoyuan Chen, Twan Lammers and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Bottleneck effects
-
An event that drastically reduces the size of a population. Bottlenecks produce a decrease in the gene pool of the population because many alleles, or gene variants, that were present in the original population are lost. Owing to the loss of genetic variation, the new population can become genetically distinct from the original population.
- Chemokines
-
A family of inducible chemoattractant cytokines that regulate the chemotaxis of tumour cells and other cell types. Chemokines also affect processes such as proliferation, migration and invasion.
- Cytokines
-
Small, secreted proteins produced by immune cells that are used in cellular communication.
- DNAzyme
-
Single-stranded DNA oligonucleotides with high catalytic activities towards specific substrates.
- Electrospun nanofibres
-
Fibres with diameters in the nanometre range created using electrospinning. Electrospinning relies on the electrostatic repulsion between surface charges to continuously draw nanofibres from a viscoelastic fluid.
- Enhanced permeability and retention effect
-
Tumours can exhibit increased permeability and retention of large molecular weight molecules or nanoparticles primarily owing to structural abnormality of tumour vasculature.
- Enzymatic suicide inactivation
-
Irreversible inhibition of the activity of an enzyme.
- Gefitinib
-
The first quinazoline-based reversible small-molecule epidermal growth factor receptor tyrosine kinase inhibitor.
- Genetic drift
-
Changes in the frequency of a genetic variant in a population owing to chance alone.
- Hypoxia
-
A subnormal concentration of oxygen. In cancer tissue, hypoxia is often the result of abnormal vasculature.
- Immune checkpoint receptors
-
Cell-surface molecules that are expressed by T cells and the normal function of which is to maintain self-tolerance and regulate the magnitude and duration of immune responses. Checkpoint receptors, including PD1 and TIM3, can be co-opted by tumours to inhibit antitumour immune responses.
- Immunoediting
-
Describes the complex relationship between a developing tumour under constant pressure from the host immune system. Cancer immunoediting consists of three phases: elimination (that is, cancer immunosurveillance), equilibrium and escape. The immune system not only protects the host against development of primary cancers but also sculpts tumour immunogenicity.
- Mechanotransduction
-
Mechanisms by which cells convert mechanical stimulus into biochemical signals.
- Microcontact printed lines
-
Microcontact printing is a method of transferring patterns of various materials such as polymers, proteins, nanoparticles and so on, onto another surface. Typically, a polydimethylsiloxane stamp is dipped in a solution of a material that has to be patterned and is brought into contact with the surface.
- Microfluidic systems
-
These small ‘plumbing’ systems deal with the accurate control and manipulation of fluids that are confined to micrometre-sized environments. This enables the supply of nutrients, oxygen and the flow of media to be precisely controlled.
- Mouse tumour xenograft models
-
Hetero-transplantation of human tumour cells into immunodeficient mice, in either the orthotopic (same organ) site or ectopic (foreign) site. Mice are typically athymic nu/nu T cell-deficient or severe combined immunodeficient, lacking B cell and T cell functions.
- Multicellular spheroids
-
Multicellular spheroids are either self-assembling or are forced to grow as 3D spherical cell clusters. They can be established from a single cell type or can be multicellular mixtures of tumour, stromal and immune cells. These aggregates can mimic tumour cell behaviour more effectively because they harbour a gradient of cells that are surface-exposed and cells that are deeply buried, thereby also establishing a gradient of nutrient and oxygen availability.
- Nanolithography
-
Set of top-down fabrication techniques that allow patterning materials and building devices with nanoscale resolution.
- Nanopipette
-
A nanoscale pipette that locally collects analytes for mass spectrometry, electrochemical and optical analysis.
- Nanoprinted scaffolds
-
Three-dimensional scaffolds with nanometre-scale features that mimic interstitial tissue or extracellular matrix. They are used either as cell migration or tumour formation platforms.
- Organoids
-
Tissue-like 3D cultures originating from human stem cells, organ-specific progenitor cells or dissociated tumour tissues, grown in a reconstituted extracellular matrix. Organoids mimic primary tissues by retaining some aspects of tissue architecture and function. Tumour-derived organoids retain the diversity and fidelity of mutational landscapes and, when transplanted into mice, reconstitute many of the histopathological features of their tumours of origin.
- Plasmonic nanoparticles
-
Metallic nanoparticles whose electron density can couple with certain wavelengths of light. Plasmonic nanoparticles exhibit interesting scattering, absorbance and coupling properties on the basis of their structures, geometries and relative positions.
- Sacrificial bioink
-
A biomaterial used as ink in 3D printing of biomimetic structures, which has gentle and reversible crosslinking properties and can be easily removed (or ‘sacrificed’) without harming the involved cells and structures.
- Self-assembled microvessels
-
Spontaneously created in vitro vascular networks driven by inherent cellular interactions between endothelial cells and stromal cells to undergo morphogenesis.
- Sentinel lymph nodes
-
The first lymph node that connects to a primary tumour site, also it is likely the first lymph node in which cancer cells spread.
- Single-walled carbon nanotubes
-
sp2-Hybridized carbon-based hollow cylindrical nanostructures that exhibit unique electronic and optical properties for intracellular and in vivo imaging and sensing.
- Solvatochromic shift
-
Phenomenon in which emission wavelength of a fluorophore changes in response to the dielectric constant of its environment.
- Surface-enhanced Raman spectroscopy
-
Highly sensitive technique that enhances the Raman scattering of molecules supported by nanostructured materials.
- Synthetic molecular recognition
-
Recognition of target analytes conferred by synthetic polymers that create a selective molecular recognition site on a nanoparticle for the molecule of interest, leading to sensitive and selective optical response.
- Vascular lumen
-
The inside space of a vessel, composed of a cord of endothelial cells.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, M., Panagiotakopoulou, M., Chen, C. et al. Micro-engineering and nano-engineering approaches to investigate tumour ecosystems. Nat Rev Cancer 23, 581–599 (2023). https://doi.org/10.1038/s41568-023-00593-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-023-00593-3