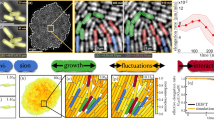
Abstract
Propagating spiral waves have been discovered in various chemical, biological and physical systems. Spiral waves in multicellular organisms are often associated with essential living functions. Although certain eukaryotic microorganisms have long been known to generate spiral waves, evidence of spiral wave pattern has been lacking in the bacterial world. Here we report the discovery of a unique form of propagating spiral waves in dense bacterial populations where cells engage in cyclic force-generating processes driven by a grappling-hook-like motile organelle called type-IV pilus motor. Specifically, we discovered that synchronization of pilus activity in the bacterial living matter leads to large-scale spatiotemporal regulation of tension force in the form of propagating spiral waves. Theoretical modelling reveals that the spiral tension waves result from non-reciprocity in cell–cell interactions. Our findings reveal a mechanism of large-scale force regulation in bacterial world and may shed light on the emergent mechanics of biofilms and microbiomes. Pilus-driven bacterial living matter also provides a mechanical active medium for studying electrical or chemical spiral waves in living systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are included within the paper and its Supplementary Information.
Code availability
The custom codes used in this study are available from the corresponding author upon request.
References
Cross, M. C. & Hohenberg, P. C. Pattern formation outside of equilibrium. Rev. Mod. Phys. 65, 851–1112 (1993).
Kuramoto, Y. Chemical Oscillations, Waves, and Turbulence (Dover Publications, 2003).
Winfree, A. T. The Geometry of Biological Time (Springer-Verlag, 2001).
Zaikin, A. N. & Zhabotinsky, A. M. Concentration wave propagation in two-dimensional liquid-phase self-oscillating system. Nature 225, 535–537 (1970).
Jakubith, S., Rotermund, H. H., Engel, W., von Oertzen, A. & Ertl, G. Spatiotemporal concentration patterns in a surface reaction: propagating and standing waves, rotating spirals, and turbulence. Phys. Rev. Lett. 65, 3013–3016 (1990).
Totz, J. F., Rode, J., Tinsley, M. R., Showalter, K. & Engel, H. Spiral wave chimera states in large populations of coupled chemical oscillators. Nat. Phys. 14, 282–285 (2018).
Davidenko, J. M., Pertsov, A. V., Salomonsz, R., Baxter, W. & Jalife, J. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature 355, 349–351 (1992).
Huang, X. et al. Spiral wave dynamics in neocortex. Neuron 68, 978–990 (2010).
Jalife, J. Ventricular fibrillation: mechanisms of initiation and maintenance. Annu. Rev. Physiol. 62, 25–50 (2000).
Narayan, S. M., Patel, J., Mulpuru, S. & Krummen, D. E. Focal impulse and rotor modulation ablation of sustaining rotors abruptly terminates persistent atrial fibrillation to sinus rhythm with elimination on follow-up: a video case study. Heart Rhythm 9, 1436–1439 (2012).
Tomchik, K. J. & Devreotes, P. N. Adenosine 3′,5′-monophosphate waves in Dictyostelium discoideum: a demonstration by isotope dilution-fluorography. Science 212, 443–446 (1981).
Takagi, S. & Ueda, T. Emergence and transitions of dynamic patterns of thickness oscillation of the plasmodium of the true slime mold Physarum polycephalum. Physica D 237, 420–427 (2008).
Mattick, J. S. Type IV Pili and twitching motility. Annu. Rev. Microbiol. 56, 289–314 (2002).
Burrows, L. L. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu. Rev. Microbiol. 66, 493–520 (2012).
Maier, B., Potter, L., So, M., Seifert Hank, S. & Sheetz Michael, P. Single pilus motor forces exceed 100 pN. Proc. Natl Acad. Sci. USA 99, 16012–16017 (2002).
Nijjer, J. et al. Mechanical forces drive a reorientation cascade leading to biofilm self-patterning. Nat. Commun. 12, 6632 (2021).
Lechleiter, J., Girard, S., Peralta, E. & Clapham, D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science 252, 123–126 (1991).
Bement, W. M. et al. Activator–inhibitor coupling between Rho signalling and actin assembly makes the cell cortex an excitable medium. Nat. Cell Biol. 17, 1471–1483 (2015).
Merz, A. J., So, M. & Sheetz, M. P. Pilus retraction powers bacterial twitching motility. Nature 407, 98–102 (2000).
Skerker, J. M. & Berg, H. C. Direct observation of extension and retraction of type IV pili. Proc. Natl Acad. Sci. USA 98, 6901–6904 (2001).
Talà, L., Fineberg, A., Kukura, P. & Persat, A. Pseudomonas aeruginosa orchestrates twitching motility by sequential control of type IV pili movements. Nat. Microbiol. 4, 774–780 (2019).
Koch, M. D., Fei, C., Wingreen, N. S., Shaevitz, J. W. & Gitai, Z. Competitive binding of independent extension and retraction motors explains the quantitative dynamics of type IV pili. Proc. Natl Acad. Sci. USA 118, e2014926118 (2021).
Strogatz, S. H. From Kuramoto to Crawford: exploring the onset of synchronization in populations of coupled oscillators. Physica D 143, 1–20 (2000).
Barkley, D. in Chemical Waves and Patterns (eds R. Kapral & K. Showalter) 163–189 (Springer, 1995).
Orans, J. et al. Crystal structure analysis reveals Pseudomonas PilY1 as an essential calcium-dependent regulator of bacterial surface motility. Proc. Natl Acad. Sci. USA 107, 1065–1070 (2010).
Denis, K. et al. Targeting type IV pili as an antivirulence strategy against invasive meningococcal disease. Nat. Microbiol. 4, 972–984 (2019).
Nolan, L. M., Cavaliere, R., Turnbull, L. & Whitchurch, C. B. Extracellular ATP inhibits twitching motility-mediated biofilm expansion by Pseudomonas aeruginosa. BMC Microbiol. 15, 55 (2015).
Maier, B. & Wong, G. C. L. How bacteria use type IV pili machinery on surfaces. Trends Microbiol. 23, 775–788 (2015).
Persat, A., Inclan Yuki, F., Engel, J. N., Stone, H. A. & Gitai, Z. Proc. Natl Acad. Sci. USA 112, 7563–7568 (2015).
Sabass, B., Koch, M. D., Liu, G., Stone, H. A. & Shaevitz, J. W. Force generation by groups of migrating bacteria. Proc. Natl Acad. Sci. USA 114, 7266–7271 (2017).
Rodesney, C. A. et al. Mechanosensing of shear by Pseudomonas aeruginosa leads to increased levels of the cyclic-di-GMP signal initiating biofilm development. Proc. Natl Acad. Sci. USA 114, 5906–5911 (2017).
Kühn, M. J. et al. Mechanotaxis directs Pseudomonas aeruginosa twitching motility. Proc. Natl Acad. Sci. USA 118, e2101759118 (2021).
Koch Matthias, D., Black Matthew, E., Han, E., Shaevitz Joshua, W. & Gitai, Z. Pseudomonas aeruginosa distinguishes surfaces by stiffness using retraction of type IV pili. Proc. Natl Acad. Sci. USA 119, e2119434119 (2022).
Ellison Courtney, K. et al. Obstruction of pilus retraction stimulates bacterial surface sensing. Science 358, 535–538 (2017).
Maier, B., Koomey, M. & Sheetz, M. P. A force-dependent switch reverses type IV pilus retraction. Proc. Natl Acad. Sci. USA 101, 10961–10966 (2004).
Kim, P.-J., Ko, T.-W., Jeong, H. & Moon, H.-T. Pattern formation in a two-dimensional array of oscillators with phase-shifted coupling. Phys. Rev. E 70, 065201 (2004).
Tanaka, M. et al. Unpinning of a spiral wave anchored around a circular obstacle by an external wave train: Common aspects of a chemical reaction and cardiomyocyte tissue. Chaos 19, 043114 (2009).
Davidsen, J., Glass, L. & Kapral, R. Topological constraints on spiral wave dynamics in spherical geometries with inhomogeneous excitability. Phys. Rev. E 70, 056203 (2004).
Zykov, V. S. Spiral wave initiation in excitable media. Philos. Trans. R. Soc. A 376, 20170379 (2018).
Nagy, M., Ákos, Z., Biro, D. & Vicsek, T. Hierarchical group dynamics in pigeon flocks. Nature 464, 890–893 (2010).
Ivlev, A. V. et al. Statistical Mechanics where Newton’s Third Law is Broken. Phys. Rev. X 5, 011035 (2015).
Lavergne, F. A., Wendehenne, H., Bäuerle, T. & Bechinger, C. Group formation and cohesion of active particles with visual perception-dependent motility. Science 364, 70–74 (2019).
Fruchart, M., Hanai, R., Littlewood, P. B. & Vitelli, V. Non-reciprocal phase transitions. Nature 592, 363–369 (2021).
You, Z., Baskaran, A. & Marchetti, M. C. Nonreciprocity as a generic route to traveling states. Proc. Natl Acad. Sci. USA 117, 19767–19772 (2020).
Uchida, N. & Golestanian, R. Synchronization and collective dynamics in a carpet of microfluidic rotors. Phys. Rev. Lett. 104, 178103 (2010).
Bull, M. S., Prakash, V. N. & Prakash, M. Ciliary flocking and emergent instabilities enable collective agility in a non-neuromuscular animal. Preprint at arXiv https://arxiv.org/abs/2107.02934 (2021).
Chakrabarti, B., Shelley, M. J. & Fürthauer, S. Collective motion and pattern formation in phase-synchronizing active fluids. Phys. Rev. Lett. 130, 128202 (2023).
Zhang, H., Hu, B., Hu, G., Ouyang, Q. & Kurths, J. Turbulence control by developing a spiral wave with a periodic signal injection in the complex Ginzburg-Landau equation. Phys. Rev. E 66, 046303 (2002).
Lee, K. et al. Metal-enhanced fluorescence to quantify bacterial adhesion. Adv. Mater. 23, H101–H104 (2011).
Hentzer, M. et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology (Reading, Eng.) 148, 87–102 (2002).
Beaussart, A. et al. Nanoscale adhesion forces of Pseudomonas aeruginosa type IV pili. ACS Nano 8, 10723–10733 (2014).
Li, Y. et al. Self-organized canals enable long range directed material transport in bacterial communities. Elife 11, e79780 (2022).
Tremblay, J. & Déziel, E. Gene expression in Pseudomonas aeruginosa swarming motility. BMC Genom. 11, 587 (2010).
Ma, L. Z., Wang, D., Liu, Y., Zhang, Z. & Wozniak, D. J. Regulation of biofilm exopolysaccharide biosynthesis and degradation in Pseudomonas aeruginosa. Ann. Rev. Microbiol. 76, 413–433 (2022).
Acknowledgements
We thank F. Jin (Shenzhen Institute of Advanced Technology), R. Kolter (Harvard University), G. A. O’Toole (Dartmouth College) and L. Yang (Southern University of Science and Technology) for generous gifts of bacterial strains. We also thank Q. Ouyang, A. Persat, S. Strogatz, L. Tang and Z. Zheng for helpful discussions. This work was supported by the Ministry of Science and Technology Most China (No. 2021YFA0910700), the Research Grants Council of Hong Kong SAR (RGC Ref. Nos. 14307822, 14307821, RFS2021-4S04 and CUHK Direct Grants) and National Natural Science Foundation of China (NSFC No. 31971182). Y. Wu acknowledges support from New Cornerstone Science Foundation through the Xplorer Prize.
Author information
Authors and Affiliations
Contributions
S.L. designed the study, performed experiments, developed the model, performed simulations, analysed and interpreted the data. Y.L. made the initial observation. Y. Wang designed the laser ablation setup. Y. Wu conceived the project, designed the study and analysed and interpreted the data. Y. Wu wrote the paper with S.L.’s input.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Physics thanks David Johnson and Michael Shelley for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Trajectories of spiral cores of propagating spiral waves at steady state.
(a) Representative spiral core trajectories acquired experimentally in artificial bacterial films of piliated P. aeruginosa (PA14 flgK::Tn5). Each trajectory lasted for ~1 hour. The range of spiral core motion is a few tens of µm and two order of magnitude smaller than the wavelength (~1 mm). The trajectories appeared random and showed no periodic meandering motion. The trajectories of different cores are labelled with different colour. The initial position of each core is set to x = 0, y = 0 for better comparison. (b) Representative spiral core trajectories in numerical simulations of the nonreciprocal coupled oscillator model with a full nonreciprocity (ε = 1) associated with main text Fig. 3f. The trajectories are plotted in the same manner as in panel A.
Extended Data Fig. 2 Mean-square displacement (MSD) and diffusivity of spiral cores measured in experiments (panel A; with 20 spiral cores) and in simulations (panel B; with 15 spiral cores).
Blue circles: MSD data. Red line: MSD fitted to \(4{Dt}\), with \(D\) being the diffusion coefficient. The collective MSD of all spiral cores was computed by segmenting spiral core trajectories with different time windows. Note that the trajectories of spiral cores obtained in experiments were corrected for their own drift, which was likely caused by cell growth during the tracking period; for the \(i\)-th core, its drift (denoted as \({v}_{d}\)) was computed by fitting the core’s own MSD (denoted as MSDi) to \({at}+{{(v}_{d}t)}^{2}\), and its drift-corrected MSD is calculated as \({\overline{{MSD}}}_{i}={MS}{D}_{i}-{\left({v}_{d}t\right)}^{2}\). Then the overall drift-corrected MSD of N spiral cores, defined as \({MS}{D}_{{all}}=\sum {\overline{{MSD}}}_{i}/N\), was fitted to \({MS}{D}_{{all}}=4{Dt}\) as shown in panel A. In panel A, \(D=6.92\)µm2/min (equivalent to 4.80 × 10−5 square wavelengths per period). In panel B, D = 9.72 × 101 µm2/min (equivalent to 6.14 × 10−4 square wavelengths per period).
Extended Data Fig. 3 Development of propagating spiral waves in disk-shaped artificial bacterial films of P. aeruginosa.
Upper row: Traces of wavefronts (identical to upper row of main text Fig. 1c). Lower row: Distributions of local instantaneous oscillation period during the spiral wave development (Methods). Color bar to the right of the lower row indicates the magnitude of instantaneous oscillation period (unit: min). Scale bar, 500 μm. Also see Movie 2.
Extended Data Fig. 4 Spatiotemporal dynamics of phase angle (upper) and angular frequency distribution (lower) in simulations of the nonreciprocal coupled oscillator model with a full nonreciprocity ε = 1.
Upper row: Phase angle distribution (identical to main text Fig. 3b). Lower row: Distributions of local instantaneous angular frequency during the spiral wave development (Methods). Color bar to the right of the lower row indicates the magnitude of instantaneous oscillation angular frequency. Also see Movie 7.
Extended Data Fig. 5 Generation of train waves by forced synchronization of oscillators in the nonreciprocal coupled oscillator model with a full nonreciprocity ε = 1.
The simulation was performed in a system with pre-existing propagating spiral waves (left panel, T = 2400 s). Starting from T = 2408 s, the oscillators near the left boundary were forced to synchronize and remained synchronized throughout the rest of the simulation, which simulated the application of a filter disk infused with drugs that trigger pilus retraction. Following the forced synchronization, train waves emanated from near the left boundary and propagated along a direction perpendicular to the boundary (right panel, T = 4000 s and T = 16000 s). The train waves gradually replaced the existing spiral waves. Also see Methods and Movie 8. The results reproduce the experimental phenomena in Movie 5 and Movie 6, supporting the notion that train waves observed in the experiments were due to forced synchronization of the pilus retraction-extension cycle.
Supplementary information
Supplementary Information
Supplementary Methods
Supplementary Video 1
Propagating spiral waves in a naturally developed piliated P. aeruginosa (PA14 flgK::Tn5) colony. This video is associated with main text Fig. 1A. Upper: time-lapse phase-contrast images. Lower: wavefronts of the propagating spiral waves (labelled as white traces) traced by the variation of light intensity in the phase-contrast images. The elapsed time is indicated by the time stamp (format: hh:mm:ss). Scale bar, 500 μm.
Supplementary Video 2
Onset and evolution of the propagating spiral waves in an artificial bacterial films of P. aeruginosa 14. This video is associated with main text Fig. 1C. Left: time-lapse phase-contrast images. Middle: Traces of wavefronts. Right: phase angle distributions of the waves. Colour bar to the right of the right panel indicate the magnitude of phase angle. The elapsed time is indicated by the time stamp (format: hh:mm:ss). Scale bar, 500 μm.
Supplementary Video 3
Local cell-density variation during the propagation of spiral waves. This video is associated with main text Fig. 2A. All cells in the artificial bacterial film were labelled by mCherry fluorescence protein. Left: time-lapse phase-contrast images. Right: time-lapse mCherry fluorescence of the bacterial film (as a proxy of cell density). The mCherry fluorescence intensity exhibits the same wave pattern that coincides with the wave observed in phase-contrast images. The elapsed time is indicated by the time stamp (format: hh:mm:ss). Scale bar, 500 μm.
Supplementary Video 4
Periodic forth-and-back displacement of cells during the propagation of spiral waves. This video is associated with main text Fig. 2B. Cells in the artificial bacterial film were labelled by either GFP (99.9%) or mCherry (0.1%) fluorescence protein. Left: time-lapse video of GFP fluorescence of the bacterial film (as a proxy of cell density). Right: time-lapse fluorescence video of mCherry-labelled cells in the bacterial film showing the periodic forth-and-back displacement of cells. The elapsed time is indicated by the time stamp (format: hh:mm:ss). Scale bar, 500 μm.
Supplementary Video 5
Effect of EGTA on the propagating spiral waves. The time-lapse phase-contrast videos show that, when a filter paper disc soaked with EGTA was placed onto an artificial bacterial film with (right panel) or without (left panel) pre-existing spiral waves, a train of waves emanated from near the edge of the filter paper disc and propagated outwards in both conditions. The elapsed time is indicated by the time stamp (format: hh:mm:ss). Scale bar, 500 μm.
Supplementary Video 6
Effect of filter discs soaked with phenothiazines and ATP on the propagating spiral waves. The time-lapse phase-contrast videos show that, when a filter paper disc soaked with trifluoperazine, thioridazine or ATP solutions (Methods) was placed onto an artificial bacterial film, a train of waves emanated from near the edge of the filter paper disc and propagated outward. By contrast, empty filter discs that were soaked with DI water or NaCl or sucrose solutions (Methods) cannot trigger train waves when placed on an artificial bacterial film (right panel). The elapsed time is indicated by the time stamp (format: hh:mm:ss). Scale bars, 500 μm.
Supplementary Video 7
Simulation results of the physical model incorporating both Kuramoto dynamics and continuum mechanics in the bacterial film. The video is associated with Fig. 3 and shows the spatial distribution of tension (left) and cell density variation (right) during the development of spiral waves. The simulation was performed in an 80 × 80 coupled oscillator system.
Supplementary Video 8
Generation of train waves by forced synchronization of oscillators in the simulation. The video is associated with Extended Data Fig. 5. The simulation was performed in a 100 × 100 coupled oscillator system. At T = 2,000 s, propagating spiral waves had already stabilized and the oscillators near the left boundary were forced to synchronize, simulating the application of a filter disk infused with drugs that trigger pilus retraction (Supplementary Videos 5 and 6). Train waves emanate from near the left boundary and propagated along a direction perpendicular to the boundary, gradually replacing the existing spiral waves.
Supplementary Video 9
Evolution of phase angle and local order parameter distributions in simulations of the nonreciprocal coupled oscillator model at different levels of non-reciprocity. The simulation was performed in a 100 × 100 coupled oscillator system. Upper: phase angle distribution at different levels of nonreciprocity; lower: local order parameter distribution corresponding to the phase angle distribution right above it, with the level of nonreciprocity indicated at the lower left corner. Starting from a disordered initial phase angle distribution, stable propagating spiral waves emerge at all levels of nonreciprocity; by contrast, transient spiral waves appeared in the case of zero nonreciprocity (ε = 0) and they eventually disappeared, leaving an almost homogeneous phase angle distribution. Simulation time steps elapsed for all panels are indicated in the lower left-most panel.
Supplementary Video 10
Effect of hollow-like spatial defect on the propagating spiral waves in the simulation. The level of nonreciprocity is indicated at the top of each simulation. Upper two rows: the videos are associated with main text Fig. 4C and show the spatial distributions of phase angle (first row) and local order parameter (second row) before and after introducing a defect (black solid circle; from T = 1,000 time steps to a simulation domain with a single pre-existing spiral core at the centre (Methods). Oscillators in the defect were inactivated and did not interact with others. The simulation was performed in a 200 × 200 coupled oscillator system. The wave dynamics was only deformed very close to the defect and the spiral core (specified by the local minima of local order parameter field) did not move. Lower two rows: the videos show the spatial distributions of phase angle (third row) and local order parameter (fourth row) before and after introducing a defect to the system (black solid circle; from T = 1,000 time steps) in simulations with pre-existing propagating spiral waves (Methods). The simulation was performed in a 100 × 100 coupled oscillator system. The wave dynamics was largely unaffected by the defect, except that at relatively low ε the spiral cores close to the defect tended to get attracted towards it and subsequently disappeared when entering the defect area.
Supplementary Video 11
Effect of hollow-like spatial defect on the propagating spiral waves in laser ablation experiment. Laser (~250 mW, 444 nm) was illuminated onto a small area of an artificial bacterial film with propagating spiral waves, which ablated cells in the illuminated area in 1 min and created a spatial defect in the area unable to mediate wave propagation. The time-lapse videos (left panel: phase-contrast; right: phase angle distribution) recorded the wave dynamics before (before 22 min 45 s) and after (starting from 24 min 59 s) the creation of spatial defect by laser ablation. The videos show that the spatial defect only affected the wave dynamics very close to the defect. The elapsed time is indicated by the time stamp (format: hh:mm:ss). Scale bars, 500 μm.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Li, Y., Wang, Y. et al. Emergence of large-scale mechanical spiral waves in bacterial living matter. Nat. Phys. (2024). https://doi.org/10.1038/s41567-024-02457-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41567-024-02457-5