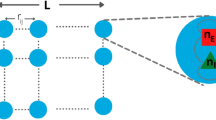
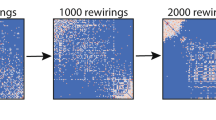
Abstract
The connections in networks of neurons are heavy-tailed, with a small number of neurons connected much more strongly than the vast majority of pairs. However, it remains unclear whether this heavy-tailed connectivity emerges from simple underlying mechanisms. Here we propose a minimal model of synaptic self-organization: connections are pruned at random, and the synaptic strength rearranges under a mixture of preferential and random dynamics. Under these generic rules, networks evolve to produce distributions of connectivity strength that are asymptotically scale-free, with a power-law exponent that depends only on the probability of preferential (rather than random) growth. Extending our model to include neuronal activity and Hebbian plasticity, we find that clustering in the network also emerges naturally. We confirm these predictions in the connectomes of several animals, suggesting that heavy-tailed and clustered connectivity may arise from general principles of network self-organization rather than mechanisms specific to individual species or systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The neuronal data analysed in this paper are openly available at github.com/ChrisWLynn/Heavy_tailed_connectivity.
Code availability
The code used to perform the analyses in this paper is openly available at github.com/ChrisWLynn/Heavy_tailed_connectivity.
References
Ho, V. M., Lee, J.-A. & Martin, K. C. The cell biology of synaptic plasticity. Science 334, 623–628 (2011).
Magee, J. C. & Grienberger, C. Synaptic plasticity forms and functions. Annu. Rev. Neurosci. 43, 95–117 (2020).
Gómez-Palacio-Schjetnan, A. & Escobar, M. L. Neurotrophins and synaptic plasticity. Curr. Top. Behav. Neurosci. 15, 117–136 (2013).
Song, S., Sjöström, P. J., Reigl, M., Nelson, S. & Chklovskii, D. B. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 3, e68 (2005).
Scheffer, L. K. et al. A connectome and analysis of the adult Drosophila central brain. eLife 9, e57443 (2020).
Feldmeyer, D., Egger, V., Lübke, J. & Sakmann, B. Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single ‘barrel’ of developing rat somatosensory cortex. J. Physiol. 521, 169–190 (1999).
Lefort, S., Tomm, C., Sarria, J.-C. F. & Petersen, C. C. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron 61, 301–316 (2009).
Ikegaya, Y. et al. Interpyramid spike transmission stabilizes the sparseness of recurrent network activity. Cereb. Cortex 23, 293–304 (2013).
Loewenstein, Y., Kuras, A. & Rumpel, S. Multiplicative dynamics underlie the emergence of the log-normal distribution of spine sizes in the neocortex in vivo. J. Neurosci. 31, 9481–9488 (2011).
Lynn, C. W. & Bassett, D. S. The physics of brain network structure, function and control. Nat. Rev. Phys. 1, 318 (2019).
Dorkenwald, S. et al. Binary and analog variation of synapses between cortical pyramidal neurons. eLife 11, e76120 (2022).
Kornfeld, J. et al. An anatomical substrate of credit assignment in reinforcement learning. Preprint at bioRxiv https://doi.org/10.1101/2020.02.18.954354 (2020).
Farashahi, S. et al. Metaplasticity as a neural substrate for adaptive learning and choice under uncertainty. Neuron 94, 401–414 (2017).
Xu, W. & Südhof, T. C. A neural circuit for memory specificity and generalization. Science 339, 1290–1295 (2013).
Mei-ling, A. J. & Griffith, L. C. CaM kinase II and visual input modulate memory formation in the neuronal circuit controlling courtship conditioning. J. Neurosci. 17, 9384–9391 (1997).
Lei, Z., Henderson, K. & Keleman, K. A neural circuit linking learning and sleep in Drosophila long-term memory. Nat. Commun. 13, 609 (2022).
Almeida, R., Barbosa, J. & Compte, A. Neural circuit basis of visuo-spatial working memory precision: a computational and behavioral study. J. Neurophysiol. 114, 1806–1818 (2015).
Helmstaedter, M. et al. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168–174 (2013).
Takemura, S.-Y. et al. A visual motion detection circuit suggested by Drosophila connectomics. Nature 500, 175–181 (2013).
Murthy, M., Fiete, I. & Laurent, G. Testing odor response stereotypy in the Drosophila mushroom body. Neuron 59, 1009–1023 (2008).
Borst, A. & Helmstaedter, M. Common circuit design in fly and mammalian motion vision. Nat. Neurosci. 18, 1067–1076 (2015).
Song, Y.-H. et al. A neural circuit for auditory dominance over visual perception. Neuron 93, 940–954 (2017).
Sokolowski, M. B. Social interactions in “simple” model systems. Neuron 65, 780–794 (2010).
Tootoonian, S., Coen, P., Kawai, R. & Murthy, M. Neural representations of courtship song in the Drosophila brain. J. Neurosci. 32, 787–798 (2012).
Varshney, L. R., Chen, B. L., Paniagua, E., Hall, D. H. & Chklovskii, D. B. Structural properties of the Caenorhabditis elegans neuronal network. PLoS Comput. Biol. 7, e1001066 (2011).
Randel, N. et al. Neuronal connectome of a sensory–motor circuit for visual navigation. eLife 3, e02730 (2014).
Yamada, T. et al. Sensory experience remodels genome architecture in neural circuit to drive motor learning. Nature 569, 708–713 (2019).
Piggott, B. J., Liu, J., Feng, Z., Wescott, S. A. & Xu, X. S. The neural circuits and synaptic mechanisms underlying motor initiation in C. elegans. Cell 147, 922–933 (2011).
Helmstaedter, M. Cellular-resolution connectomics: challenges of dense neural circuit reconstruction. Nat. Methods 10, 501–507 (2013).
White, J. G., Southgate, E., Thomson, J. N. & Brenner, S. et al. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B 314, 1–340 (1986).
Butz, M., Wörgötter, F. & van Ooyen, A. Activity-dependent structural plasticity. Brain Res. Rev. 60, 287–305 (2009).
Stringer, C., Pachitariu, M., Steinmetz, N., Carandini, M. & Harris, K. D. High-dimensional geometry of population responses in visual cortex. Nature 571, 361–365 (2019).
Dorogovtsev, S. N. & Mendes, J. F. Evolution of networks. Adv. Phys. 51, 1079–1187 (2002).
Lynn, C. W., Holmes, C. M. & Palmer, S. E. Emergent scale-free networks. Preprint at arXiv https://doi.org/10.48550/arXiv.2210.06453 (2022).
Caporale, N. & Dan, Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu. Rev. Neurosci. 31, 25–46 (2008).
Markram, H., Lübke, J., Frotscher, M. & Sakmann, B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275, 213–215 (1997).
Liu, Y.-Y., Slotine, J.-J. & Barabási, A.-L. Controllability of complex networks. Nature 473, 167–173 (2011).
Lynn, C. W., Papadopoulos, L., Kahn, A. E. & Bassett, D. S. Human information processing in complex networks. Nat. Phys. 16, 965–973 (2020).
Lynn, C. W. & Bassett, D. S. Quantifying the compressibility of complex networks. Proc. Natl Acad. Sci. USA 118, e2023473118 (2021).
Yuste, R. From the neuron doctrine to neural networks. Nat. Rev. Neurosci. 16, 487–497 (2015).
Bianconi, G. Mean field solution of the Ising model on a Barabási–Albert network. Phys. Lett. A 303, 166–168 (2002).
Lynn, C. W. & Lee, D. D. in Advances in Neural Information Processing Systems (eds Lee, D. et al.) 2495–2503 (2016).
Aguilera, M., Moosavi, S. A. & Shimazaki, H. A unifying framework for mean-field theories of asymmetric kinetic Ising systems. Nat. Commun. 12, 1197 (2021).
Schneidman, E., Berry, M. J., Segev, R. & Bialek, W. Weak pairwise correlations imply strongly correlated network states in a neural population. Nature 440, 1007 (2006).
Tkačik, G. et al. Searching for collective behavior in a large network of sensory neurons. PLoS Comput. Biol. 10, e1003408 (2014).
Kim, J. Z., Lu, Z., Nozari, E., Pappas, G. J. & Bassett, D. S. Teaching recurrent neural networks to infer global temporal structure from local examples. Nat. Mach. Intell. 3, 316–323 (2021).
Bentley, B. et al. The multilayer connectome of Caenorhabditis elegans. PLoS Comput. Biol. 12, e1005283 (2016).
Bassett, D. S. & Sporns, O. Network neuroscience. Nat. Neurosci. 20, 353–364 (2017).
Stiso, J. & Bassett, D. S. Spatial embedding imposes constraints on neuronal network architectures. Trends Cogn. Sci. 22, 1127–1142 (2018).
Morrison, A., Aertsen, A. & Diesmann, M. Spike-timing-dependent plasticity in balanced random networks. Neural Comput. 19, 1437–1467 (2007).
Effenberger, F., Jost, J. & Levina, A. Self-organization in balanced state networks by STDP and homeostatic plasticity. PLoS Comput. Biol. 11, e1004420 (2015).
Yang, G., Pan, F. & Gan, W.-B. Stably maintained dendritic spines are associated with lifelong memories. Nature 462, 920–924 (2009).
Oh, W. C., Hill, T. C. & Zito, K. Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening. Proc. Natl Acd. Sci. USA 110, E305–E312 (2013).
Brunel, N. Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. J. Comput. Neurosci. 8, 183–208 (2000).
Mitchell, S. M., Lange, S. & Brus, H. Gendered citation patterns in international relations journals. Int. Stud. Perspect. 14, 485–492 (2013).
Dion, M. L., Sumner, J. L. & Mitchell, S. M. Gendered citation patterns across political science and social science methodology fields. Polit. Anal. 26, 312–327 (2018).
Caplar, N., Tacchella, S. & Birrer, S. Quantitative evaluation of gender bias in astronomical publications from citation counts. Nat. Astron. 1, 0141 (2017).
Dworkin, J. D. et al. The extent and drivers of gender imbalance in neuroscience reference lists. Nat. Neurosci. 23, 918–926 (2020).
Bertolero, M. A. et al. Racial and ethnic imbalance in neuroscience reference lists and intersections with gender. Preprint at bioRxiv https://doi.org/10.1101/2020.10.12.336230 (2020).
Teich, E. G. et al. Citation inequity and gendered citation practices in contemporary physics. Nat. Phys. 18, 1161–1170 (2022).
Acknowledgements
We thank D. J. Schwab and L. Papadopoulos for discussions and feedback and P. Kratsios for help with illustrations. This work was supported in part by the National Science Foundation, through the Center for the Physics of Biological Function (PHY–1734030) and a Graduate Research Fellowship (C.M.H.); by the James S. McDonnell Foundation through a Postdoctoral Fellowship Award (C.W.L.); and by the National Institutes of Health BRAIN initiative (R01EB026943).
Author information
Authors and Affiliations
Contributions
C.W.L. and S.E.P. conceived the project. C.W.L. designed the models, and C.W.L. and C.M.H. performed the analysis with input from S.E.P. C.W.L. wrote the paper and Supplementary Information, and C.M.H. and S.E.P. edited the paper and Supplementary Information.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Physics thanks Manfred Kitzbichler, Dietmar Plenz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3 and Discussion.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lynn, C.W., Holmes, C.M. & Palmer, S.E. Heavy-tailed neuronal connectivity arises from Hebbian self-organization. Nat. Phys. 20, 484–491 (2024). https://doi.org/10.1038/s41567-023-02332-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-023-02332-9