Abstract
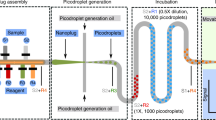
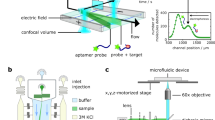
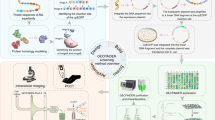
The analysis of proteins at the single-molecule level reveals heterogeneous behaviours that are masked in ensemble-averaged techniques. The digital quantification of enzymes traditionally involves the observation and counting of single molecules partitioned into microcompartments via the conversion of a profluorescent substrate. This strategy, based on linear signal amplification, is limited to a few enzymes with sufficiently high turnover rate. Here we show that combining the sensitivity of an exponential molecular amplifier with the modularity of DNA–enzyme circuits and droplet readout makes it possible to specifically detect, at the single-molecule level, virtually any D(R)NA-related enzymatic activity. This strategy, denoted digital PUMA (Programmable Ultrasensitive Molecular Amplifier), is validated for more than a dozen different enzymes, including many with slow catalytic rate, and down to the extreme limit of apparent single turnover for Streptococcus pyogenes Cas9. Digital counting uniquely yields absolute molar quantification and reveals a large fraction of inactive catalysts in all tested commercial preparations. By monitoring the amplification reaction from single enzyme molecules in real time, we also extract the distribution of activity among the catalyst population, revealing alternative inactivation pathways under various stresses. Our approach dramatically expands the number of enzymes that can benefit from quantification and functional analysis at single-molecule resolution. We anticipate digital PUMA will serve as a versatile framework for accurate enzyme quantification in diagnosis or biotechnological applications. These digital assays may also be utilized to study the origin of protein functional heterogeneity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper and its Supplementary Information. All nucleic acid sequences and experimental conditions are available in Supplementary Tables 1 and 3. Unprocessed data related to Figs. 1–4 are provided in the source data file. Raw data (droplet coordinates and fluorescence for each time point) from Figs. 5 and 6 can be accessed at the following publicly accessible repository: https://doi.org/10.5281/zenodo.10455918, https://doi.org/10.5281/zenodo.10455829 and https://doi.org/10.5281/zenodo.10455612.
Code availability
The Mathematica code used for droplet analysis is available via GitHub at the following link: https://github.com/GuGi75/Droplet-analysis
References
Greenough, L. et al. Adapting capillary gel electrophoresis as a sensitive, high-throughput method to accelerate characterization of nucleic acid metabolic enzymes. Nucleic Acids Res. 44, e15 (2016).
Farag, N. et al. Folding-upon-repair DNA nanoswitches for monitoring the activity of DNA repair enzymes. Angew. Chem. 133, 7359–7365 (2021).
Luo, X. & Hsing, I.-M. Immobilization-free electrochemical DNA polymerase assay. Electroanalysis 23, 923–926 (2011).
Boehr, D. D., Nussinov, R. & Wright, P. E. The role of dynamic conformational ensembles in biomolecular recognition. Nat. Chem. Biol. 5, 789–796 (2009).
Henzler-Wildman, K. & Kern, D. Dynamic personalities of proteins. Nature 450, 964–972 (2007).
Leveson-Gower, R. B., Mayer, C. & Roelfes, G. The importance of catalytic promiscuity for enzyme design and evolution. Nat. Rev. Chem. 3, 687–705 (2019).
Rotman, B. Measurement of activity of single molecules of β-d-galactosidase. Proc. Natl Acad. Sci. USA 47, 1981–1991 (1961).
Vogelstein, B. & Kinzler, K. W. Digital PCR. Proc. Natl Acad. Sci. USA 96, 9236–9241 (1999).
Rondelez, Y. et al. Microfabricated arrays of femtoliter chambers allow single molecule enzymology. Nat. Biotechnol. 23, 361–365 (2005).
Ono, T., Ichiki, T. & Noji, H. Digital enzyme assay using attoliter droplet array. Analyst 143, 4923–4929 (2018).
Guan, Z. et al. A highly parallel microfluidic droplet method enabling single-molecule counting for digital enzyme detection. Biomicrofluidics 8, 014110 (2014).
Rojek, M. J. & Walt, D. R. Observing single enzyme molecules interconvert between activity states upon heating. PLoS One 9, e86224 (2014).
Rissin, D. M. & Walt, D. R. Digital concentration readout of single enzyme molecules using femtoliter arrays and Poisson statistics. Nano Lett. 6, 520–523 (2006).
Liebherr, R. B. et al. Three-in-one enzyme assay based on single molecule detection in femtoliter arrays. Anal. Bioanal. Chem. 407, 7443–7452 (2015).
Obayashi, Y., Iino, R. & Noji, H. A single-molecule digital enzyme assay using alkaline phosphatase with a cumarin-based fluorogenic substrate. Analyst 140, 5065–5073 (2015).
Gorris, H. H., Rissin, D. M. & Walt, D. R. Stochastic inhibitor release and binding from single-enzyme molecules. Proc. Natl Acad. Sci. USA 104, 17680–17685 (2007).
English, B. P. et al. Ever-fluctuating single enzyme molecules: Michaelis–Menten equation revisited. Nat. Chem. Biol. 2, 87–94 (2006).
Hsin, T.-M. & Yeung, E. S. Single-molecule reactions in liposomes. Angew. Chem. Int. Ed. 46, 8032–8035 (2007).
Ueno, H., Kato, M., Minagawa, Y., Hirose, Y. & Noji, H. Elucidation and control of low and high active populations of alkaline phosphatase molecules for quantitative digital bioassay. Protein Sci. 30, 1628–1639 (2021).
Jiang, Y., Li, X. & Walt, D. R. Single-molecule analysis determines isozymes of human alkaline phosphatase in serum. Angew. Chem. Int. Ed. 59, 18010–18015 (2020).
Craig, D. B., Arriaga, E. A., Wong, J. C. Y., Lu, H. & Dovichi, N. J. Studies on Single alkaline phosphatase molecules: reaction rate and activation energy of a reaction catalyzed by a single molecule and the effect of thermal denaturation—the death of an enzyme. J. Am. Chem. Soc. 118, 5245–5253 (1996).
Sakuma, M. et al. Genetic perturbation alters functional substates in alkaline phosphatase. J. Am. Chem. Soc. 145, 2806–2814 (2023).
Gorris, H. H. & Walt, D. R. Mechanistic aspects of horseradish peroxidase elucidated through single-molecule studies. J. Am. Chem. Soc. 131, 6277–6282 (2009).
Ehrl, B. N., Liebherr, R. B. & Gorris, H. H. Single molecule kinetics of horseradish peroxidase exposed in large arrays of femtoliter-sized fused silica chambers. Analyst 138, 4260–4265 (2013).
Comellas-Aragonès, M. et al. A virus-based single-enzyme nanoreactor. Nat. Nanotechnol. 2, 635–639 (2007).
Liebherr, R. B., Renner, M. & Gorris, H. H. A single molecule perspective on the functional diversity of in vitro evolved β-glucuronidase. J. Am. Chem. Soc. 136, 5949–5955 (2014).
Jiang, Y. et al. Single-molecule mechanistic study of enzyme hysteresis. ACS Cent. Sci. 5, 1691–1698 (2019).
Watanabe, R., Sakuragi, T., Noji, H. & Nagata, S. Single-molecule analysis of phospholipid scrambling by TMEM16F. Biophys. J. 114, 558a (2018).
Tan, W. & Yeung, E. S. Monitoring the reactions of single enzyme molecules and single metal ions. Anal. Chem. 69, 4242–4248 (1997).
Sakakihara, S., Araki, S., Iino, R. & Noji, H. A single-molecule enzymatic assay in a directly accessible femtoliter droplet array. Lab Chip 10, 3355–3362 (2010).
Watanabe, R. et al. Arrayed lipid bilayer chambers allow single-molecule analysis of membrane transporter activity. Nat. Commun. 5, 4519 (2014).
Ueno, H., Sano, M., Hara, M. & Noji, H. Digital cascade assays for ADP- or ATP-producing enzymes using a femtoliter reactor array device. ACS Sens. 8, 3400–3407 (2023).
Noji, H., Minagawa, Y. & Ueno, H. Enzyme-based digital bioassay technology—key strategies and future perspectives. Lab Chip 22, 3092–3109 (2022).
Cox, K. J., Subramanian, H. K. K., Samaniego, C. C., Franco, E. & Choudhary, A. A universal method for sensitive and cell-free detection of CRISPR-associated nucleases. Chem. Sci. 10, 2653–2662 (2019).
Sternberg, S. H., Redding, S., Jinek, M., Greene, E. C. & Doudna, J. A. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 507, 62–67 (2014).
Montagne, K., Gines, G., Fujii, T. & Rondelez, Y. Boosting functionality of synthetic DNA circuits with tailored deactivation. Nat. Commun. 7, 13474 (2016).
Gines, G. et al. Isothermal digital detection of microRNA using background-free molecular circuit. Sci. Adv. 6, eaay5952 (2020).
Shenshin, V. A., Lescanne, C., Gines, G. & Rondelez, Y. A small-molecule chemical interface for molecular programs. Nucleic Acids Res. 49, 7765–7774 (2021).
Okumura, S. et al. Nonlinear decision-making with enzymatic neural networks. Nature 610, 496–501 (2022).
Li, Y. et al. Ultrasensitive isothermal detection of SARS-CoV-2 based on self-priming hairpin-utilized amplification of the G-rich sequence. Anal. Chem. 94, 17448–17455 (2022).
Richardson, C. D., Ray, G. J., DeWitt, M. A., Curie, G. L. & Corn, J. E. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat. Biotechnol. 34, 339–344 (2016).
Raper, A. T., Stephenson, A. A. & Suo, Z. Functional insights revealed by the kinetic mechanism of CRISPR/Cas9. J. Am. Chem. Soc. 140, 2971–2984 (2018).
Phaneuf, C. R. et al. Ultrasensitive multi-species detection of CRISPR-Cas9 by a portable centrifugal microfluidic platform. Anal. Methods 11, 559–565 (2019).
Zhang, X.-P. & Heyer, W.-D. in DNA Recombination: Methods and Protocols (ed. Tsubouchi, H.) 329–343 (Humana Press, 2011); https://doi.org/10.1007/978-1-61779-129-1_19
Tanford, C. in Advances in Protein Chemistry vol. 23 (eds. Anfinsen, C. B. et al.) 121–282 (Academic Press, 1968).
Berlett, B. S. & Stadtman, E. R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 272, 20313–20316 (1997).
Liu, G., Lin, Q., Jin, S. & Gao, C. The CRISPR-Cas toolbox and gene editing technologies. Mol. Cell 82, 333–347 (2022).
Phan, Q. A., Truong, L. B., Medina-Cruz, D., Dincer, C. & Mostafavi, E. CRISPR/Cas-powered nanobiosensors for diagnostics. Biosens. Bioelectron. 197, 113732 (2022).
Abate, A. R., Hung, T., Mary, P., Agresti, J. J. & Weitz, D. A. High-throughput injection with microfluidics using picoinjectors. Proc. Natl Acad. Sci. USA 107, 19163–19166 (2010).
Mazutis, L. & Griffiths, A. D. Selective droplet coalescence using microfluidic systems. Lab Chip 12, 1800–1806 (2012).
Mattox, A. K. et al. Applications of liquid biopsies for cancer. Sci. Transl. Med. 11, eaay1984 (2019).
Heitzer, E., Haque, I. S., Roberts, C. E. S. & Speicher, M. R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 20, 71–88 (2019).
Abbotts, R. & Madhusudan, S. Human AP endonuclease 1 (APE1): from mechanistic insights to druggable target in cancer. Cancer Treat. Rev. 36, 425–435 (2010).
Collins, A. R. & Gaivão, I. DNA base excision repair as a biomarker in molecular epidemiology studies. Mol. Asp. Med. 28, 307–322 (2007).
Zaher, D. M. et al. Recent advances with alkaline phosphatase isoenzymes and their inhibitors. Arch. Pharm. 353, e2000011 (2020).
Sachsenhauser, V. & Bardwell, J. C. Directed evolution to improve protein folding in vivo. Curr. Opin. Struct. Biol. 48, 117–123 (2018).
Dramé-Maigné, A. et al. In vitro enzyme self-selection using molecular programs. ACS Synth. Biol. https://doi.org/10.1021/acssynbio.3c00385 (2024).
Xue, Q. & Yeung, E. S. Differences in the chemical reactivity of individual molecules of an enzyme. Nature 373, 681–683 (1995).
Craig, D. B. et al. Differences in the average single molecule activities of E. coli β-galactosidase: effect of source, enzyme molecule age and temperature of induction. J. Protein Chem. 22, 555–561 (2003).
Tawfik, D. S. Messy biology and the origins of evolutionary innovations. Nat. Chem. Biol. 6, 692–696 (2010).
Willensdorfer, M., Bürger, R. & Nowak, M. A. Phenotypic mutation rates and the abundance of abnormal proteins in yeast. PLoS Comput. Biol. 3, e203 (2007).
Yamagata, A., Masui, R., Kakuta, Y., Kuramitsu, S. & Fukuyama, K. Overexpression, purification and characterization of RecJ protein from Thermus thermophilus HB8 and its core domain. Nucleic Acids Res. 29, 4617–4624 (2001).
Menezes, R., Dramé-Maigné, A., Taly, V., Rondelez, Y. & Gines, G. Streamlined digital bioassays with a 3D printed sample changer. Analyst 145, 572–581 (2019).
Lobato-Dauzier, N. et al. Silicon chambers for enhanced incubation and imaging of microfluidic droplets. Lab Chip 23, 2854–2865 (2023).
Pekin, D. et al. Quantitative and sensitive detection of rare mutations using droplet-based microfluidics. Lab Chip 11, 2156–2166 (2011).
Acknowledgements
We thank the R&D department of New England Biolabs for providing us with the enzyme concentrations. We thank N. Lobato-Dauzier, A. Genot and the platform FEMTO-ST (CNRS, Besançon) for providing the silicon-made incubation chambers. This work was supported by the European Research Council under the framework program H2020 for research and innovation (grant projects MoP-MiP, number 949493 and ProFF, number 647275) and ANR grant number 243063 MoBiDYC.
Author information
Authors and Affiliations
Contributions
G.G. and Y.R. conceived the study and contributed to the design of experiments. G.G., R.E. A.D.-M. and N.L. performed the experiments. A.B. designed the spy.Cas9 assay and produced the sgRNA. G.G., Y.R. and R.E. contributed to data analysis and interpretation. G.G. drafted the paper and all authors provided feedback.
Corresponding author
Ethics declarations
Competing interests
G.G. and Y.R. are listed as inventors on a patent assigned to the CNRS, INSERM, ESPCI Paris, Université de recherche PSL and Université Paris Cité. All other authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Hans-Heiner Gorris, Fabrice Gielen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Notes 1–3, Figs. 1–33 and Tables 1–4.
Source data
Source Data Fig. 1
Unprocessed amplification time traces.
Source Data Fig. 2
Unprocessed amplification time traces.
Source Data Fig. 3
Unprocessed amplification time traces.
Source Data Fig. 4
Droplets analysis source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gines, G., Espada, R., Dramé-Maigné, A. et al. Functional analysis of single enzymes combining programmable molecular circuits with droplet-based microfluidics. Nat. Nanotechnol. (2024). https://doi.org/10.1038/s41565-024-01617-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41565-024-01617-1