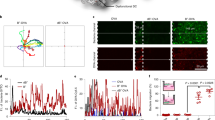
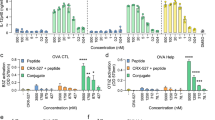
Abstract
Trained immunity enhances the responsiveness of immune cells to subsequent infections or vaccinations. Here we demonstrate that pre-vaccination with bacteria-derived outer-membrane vesicles, which contain large amounts of pathogen-associated molecular patterns, can be used to potentiate, and enhance, tumour vaccination by trained immunity. Intraperitoneal administration of these outer-membrane vesicles to mice activates inflammasome signalling pathways and induces interleukin-1β secretion. The elevated interleukin-1β increases the generation of antigen-presenting cell progenitors. This results in increased immune response when tumour antigens are delivered, and increases tumour-antigen-specific T-cell activation. This trained immunity increased protection from tumour challenge in two distinct cancer models.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. There are no data from third-party or publicly available datasets. The accession number for the raw data files for the transcriptome and ATAC sequencing reported in this paper is NCBI PRJNA861796. Other source data that support the findings of this study are available from the corresponding authors upon reasonable request. Source data for Figs. 1–5 are available in separate source data files for Figs. 1b,e–g, 2a–c,e–g,i,k, 3a–b,e,d, g-i, 4b–c,e,g–j and 5b,e,g–j, respectively. Source data for Extended Data Fig. 1 are available in separate source data files for Extended Data Fig. 1a–b,d–f. Source data for PDFs 1–3 are available in separate source data files for Figs. 1a, 4c,d and 5c,d. Supplementary XLSs 1–43 are available in separate supplementary files for Supplementary Figs. 1b, 3b,c, 4b,c, 6b,d–f, 7b–d, 8c–g, 11a–c, 16b–e, 17b,c, 18b, 21, 23b,c, 24, 25a,b, 26b–e, 27, 28b,c, 29a–d and 30a,b, respectively. Supplementary Fig. 1 and Supplementary PDFs 1–5 are available in separate supplementary files for Supplementary Figs. 18a, 19, 20, 28a,c and 29d, respectively. Source data are provided with this paper.
References
Saxena, M., van der, Burg, S. H., Melief, C. J. M. & Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 21, 360–378 (2021).
Zhang, L. et al. Nanovaccine’s rapid induction of anti-tumor immunity significantly improves malignant cancer immunotherapy. Nano Today 35, 100923 (2020).
Gardner, A. & Ruffell, B. Dendritic cells and cancer immunity. Trends Immunol. 37, 855–865 (2016).
Yang, W., Zhou, Z., Lau, J., Hu, S. & Chen, X. Functional T cell activation by smart nanosystems for effective cancer immunotherapy. Nano Today 27, 28–47 (2019).
Lee, D. Y., Huntoon, K., Wang, Y., Jiang, W. & Kim, B. Y. S. Harnessing innate immunity using biomaterials for cancer immunotherapy. Adv. Mater. 33, 2007576 (2021).
Liang, J. & Zhao, X. Nanomaterial-based delivery vehicles for therapeutic cancer vaccine development. Cancer Biol. Med. 18, 352–371 (2021).
Liu, G., Zhu, M., Zhao, X. & Nie, G. Nanotechnology-empowered vaccine delivery for enhancing CD8+ T cells-mediated cellular immunity. Adv. Drug. Deliv. Rev. 176, 113889 (2021).
Cabral, M. G. The phagocytic capacity and immunological potency of human dendritic cells is improved by α2,6-sialic acid deficiency. Immunology 138, 235–245 (2013).
Zhu, N. et al. Comparison of immunoregulatory effects of polysaccharides from three natural herbs and cellular uptake in dendritic cells. Int. J. Biol. Macromol. 93, 940–951 (2016).
Patin, E. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat. Immunol. 19, 302–314 (2018).
Dominguez-Andres, J. & Netea, M. G. Long-term reprogramming of the innate immune system. J. Leukoc. Biol. 105, 329–338 (2019).
Netea, M. G., Quintin, J. & van der Meer, J. W. Trained immunity: a memory for innate host defense. Cell Host Microbe 9, 355–361 (2011).
Netea, M. G., Schlitzer, A., Placek, K., Joosten, L. A. B. & Schultze, J. L. Innate and adaptive immune memory: an evolutionary continuum in the host’s response to pathogens. Cell Host Microbe 25, 13–26 (2019).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Netea, M. G. et al. Trained immunity: a program of innate immune memory in health and disease. Science 352, aaf1098 (2016).
Kaufmann, E. et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172, 176–190.e19 (2018).
Mitroulis, I. et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172, 147–161.e12 (2018).
Jentho, E. et al. Trained innate immunity, long-lasting epigenetic modulation, and skewed myelopoiesis by heme. Proc. Natl Acad. Sci. USA 118, e2102698118 (2021).
Bekkering, S., Dominguez-Andres, J., Joosten, L. A. B., Riksen, N. P. & Netea, M. G. Trained immunity: reprogramming innate immunity in health and disease. Annu. Rev. Immunol. 39, 667–693 (2021).
Kleinnijenhuis, J. et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate. Immunol. 6, 152–158 (2014).
Novakovic, B. et al. β-glucan reverses the epigenetic state of LPS-induced immunological tolerance. Cell 167, 1354–1368.e14 (2016).
Cirovic, B. et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe 28, 322–334.e5 (2020).
Christ, A. et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172, 162–175.e14 (2018).
Crisan, T. O. et al. Uric acid priming in human monocytes is driven by the AKT-PRAS40 autophagy pathway. Proc. Natl Acad. Sci. USA 114, 5485–5490 (2017).
Teufel, L. U., Arts, R. J. W., Netea, M. G., Dinarello, C. A. & Joosten, L. A. B. IL-1 family cytokines as drivers and inhibitors of trained immunity. Cytokine 150, 155773 (2022).
Moorlag, S. J. C. F. M., Roring, R. J., Joosten, L. A. B. & Netea, M. G. The role of the interleukin-1 family in trained immunity. Immunol. Rev. 281, 28–39 (2018).
Swanson, K. V., Deng, M. & Ting, J. PY. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 (2019).
Zhao, Y. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600 (2011).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Priem, B. et al. Trained immunity-promoting nanobiologic therapy suppresses tumor growth and potentiates checkpoint inhibition. Cell 183, 786–801.e19 (2020).
Schwechheimer, C. & Kuehn, M. J. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 13, 605–619 (2015).
Li, M. et al. Nanovaccines integrating endogenous antigens and pathogenic adjuvants elicit potent antitumor immunity. Nano Today 35, 101007 (2020).
Yue, Y. et al. Antigen-bearing outer membrane vesicles as tumour vaccines produced in situ by ingested genetically engineered bacteria. Nat. Biomed. Eng. 6, 898–909 (2022).
Li, Y. et al. Rapid surface display of mRNA antigens by bacteria-derived outer membrane vesicles for a personalized tumor vaccine. Adv. Mater. 34, e2109984 (2022).
Cheng, K. et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via plug-and-display technology. Nat. Commun. 12, 2041 (2021).
Liang, J. et al. Personalized cancer vaccines from bacteria-derived outer membrane vesicles with antibody-mediated persistent uptake by dendritic cells. Fundamental Res. 2, 23–36 (2022).
Rathinam, V. A. K., Zhao, Y. & Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 20, 527–533 (2019).
Vanaja, S. K. et al. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 165, 1106–1119 (2016).
Youngblood, B. et al. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature 552, 404–409 (2017).
Thompson, J. C. et al. Gene signature of antigen processing and presentation machinery predicts response to checkpoint blockade in non-small cell lung cancer (NSCLC) and melanoma. J. Immunother. Cancer 8, e000974 (2020).
Kelly, A. & Trowsdale, J. Genetics of antigen processing and presentation. Immunogenetics 71, 161–170 (2019).
Mangold, C. A. et al. CNS-wide sexually dimorphic induction of the major histocompatibility complex 1 pathway with aging. J. Gerontol. A. Biol. Sci. Med. Sci. 72, 16–29 (2017).
Vasu, C. et al. CD80 and CD86 C domains play an important role in receptor binding and co-stimulatory properties. Int. Immunol. 15, 167–175 (2003).
Tay, M. Z., Poh, C. M., Renia, L., MacAry, P. A. & Ng, L. F. P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 20, 363–374 (2020).
Xu, B. et al. CCR9 and CCL25: a review of their roles in tumor promotion. J. Cell. Physiol. 235, 9121–9132 (2020).
Fischer, A. et al. ZAP70: a master regulator of adaptive immunity. Semin. Immunopathol. 32, 107–116 (2010).
Lin, Q. et al. Epigenetic program and transcription factor circuitry of dendritic cell development. Nucleic Acids Res. 43, 9680–9693 (2015).
Karrich, J. J. et al. The transcription factor Spi-B regulates human plasmacytoid dendritic cell survival through direct induction of the antiapoptotic gene BCL2-A1. Blood 119, 5191–5200 (2012).
Schotte, R., Nagasawa, M., Weijer, K., Spits, H. & Blom, B. The ETS transcription factor Spi-B is required for human plasmacytoid dendritic cell development. J. Exp. Med. 200, 1503–1509 (2004).
Kanada, S. et al. Critical role of transcription factor PU.1 in the expression of CD80 and CD86 on dendritic cells. Blood 117, 2211–2222 (2011).
Cheng, S. et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345, 1250684 (2014).
Dinarello, C. A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 281, 8–27 (2018).
Gillard, J. et al. BCG-induced trained immunity enhances acellular pertussis vaccination responses in an explorative randomized clinical trial. NPJ Vaccines 7, 21 (2022).
Acevedo, R. et al. Bacterial outer membrane vesicles and vaccine applications. Front. Immunol. 5, 121 (2014).
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2021YFA0909900 and 2022YFB3808100, X.Z.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB36000000, G.N.), the CAS Project for Young Scientists in Basic Research (YSBR-010, X.Z.), the Beijing Natural Science Foundation (Z200020, X.Z.) and the National Natural Science Foundation of China (32222045 and 32171384, X.Z.). In addition, we would like to thank Wenjuan Zhang for her help on cryo-EM sampling and imaging at the Cryo-electron Microscopy Platform, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences (IGDB).
Author information
Authors and Affiliations
Contributions
G.L., N.M., K.C., X.Z. and G.N. designed the research. G.L., N.M., K.C., Q.F., X.M., Y.Y., Y.L., T.Z., X.G., J.L., L.Z. and X.W. performed the research. Z.R. and Y.-X.F. provided professional support for animal studies. All authors analysed and interpreted the data. G.L., X.Z. and G.N. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Willem Mulder and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 OMVs improved the antitumour efficacy of subsequent tumour vaccinations.
(a) The antitumour efficacy enhancement of subsequent tumour vaccinations in pulmonary metastatic B16-OVA tumour model mice (n = 6 biologically independent mice per group). (b) Antitumour efficacy enhancement of subsequent tumour vaccinations in subcutaneous MC38 tumour models mice (n = 8 biologically independent mice per group). (c–f) The antitumour efficacy enhancement of subsequent tumour vaccinations in long-term immune memory models. (C) Schematic illustration of the experiment schedule and group information. (D) Proportions of effector memory T cells (Tem, CD3+CD8+CD44+CD62L−) and central memory T cells (Tcm, CD3+CD8+CD44+CD62L+) in splenocytes on day 60, as determined by flow cytometry (n = 6 biologically independent mice per group). (E) Growth curves of the subcutaneous tumours in each mouse challenged by subcutaneous inoculation with MC38 cells (n = 10). (F) Survival curves of the mice from day 90 (n = 10). The data were processed on GraphPad Prism software (v8.3.0.538) and are presented as the mean ± SD. The P values were determined using one-way ANOVA with a Tukey post hoc test. Survival significance was analysed by the two-sided log-rank test. ns, no significance; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Supplementary information
Supplementary Information
Table of contents, Supplementary Discussion, Figs. 1–35 and Tables 1–3.
Supplementary Table 1
Raw dataset for Supplementary Fig. 1b.
Supplementary Table 2
Raw dataset for Supplementary Fig. 3b.
Supplementary Table 3
Raw dataset for Supplementary Fig. 3c.
Supplementary Table 4
Raw dataset for Supplementary Fig. 4b.
Supplementary Table 5
Raw dataset for Supplementary Fig. 4c.
Supplementary Table 6
Raw dataset for Supplementary Fig. 6b.
Supplementary Table 7
Raw dataset for Supplementary Fig. 6d.
Supplementary Table 8
Raw dataset for Supplementary Fig. 6e.
Supplementary Table 9
Raw dataset for Supplementary Fig. 6f.
Supplementary Table 10
Raw dataset for Supplementary Fig. 7b.
Supplementary Table 11
Raw dataset for Supplementary Fig. 7c.
Supplementary Table 12
Raw dataset for Supplementary Fig. 7d.
Supplementary Table 13
Raw dataset for Supplementary Fig. 8c.
Supplementary Table 14
Raw dataset for Supplementary Fig. 8d.
Supplementary Table 15
Raw dataset for Supplementary Fig. 8e.
Supplementary Table 16
Raw dataset for Supplementary Fig. 8f.
Supplementary Table 17
Raw dataset for Supplementary Fig. 8g.
Supplementary Table 18
Raw dataset for Supplementary Fig. 11a.
Supplementary Table 19
Raw dataset for Supplementary Fig. 11b.
Supplementary Table 20
Raw dataset for Supplementary Fig. 11c.
Supplementary Table 21
Raw dataset for Supplementary Fig. 16b.
Supplementary Table 22
Raw dataset for Supplementary Fig. 16c.
Supplementary Table 23
Raw dataset for Supplementary Fig. 16d.
Supplementary Table 24
Raw dataset for Supplementary Fig. 16e.
Supplementary Table 25
Raw dataset for Supplementary Fig. 17b.
Supplementary Table 26
Raw dataset for Supplementary Fig. 17c.
Supplementary Table 27
Raw dataset for Supplementary Fig. 18b.
Supplementary Table 28
Raw dataset for Supplementary Fig. 21.
Supplementary Table 29
Raw dataset for Supplementary Fig. 23b.
Supplementary Table 30
Raw dataset for Supplementary Fig. 23c.
Supplementary Table 31
Raw dataset for Supplementary Fig. 24.
Supplementary Table 32
Raw dataset for Supplementary Fig. 25a.
Supplementary Table 33
Raw dataset for Supplementary Fig. 25b.
Supplementary Table 34
Raw dataset for Supplementary Fig. 26b.
Supplementary Table 35
Raw dataset for Supplementary Fig. 26c.
Supplementary Table 36
Raw dataset for Supplementary Fig. 26d.
Supplementary Table 37
Raw dataset for Supplementary Fig. 26e.
Supplementary Table 38
Raw dataset for Supplementary Fig. 27.
Supplementary Table 39
Raw dataset for Supplementary Fig. 28b,c.
Supplementary Table 40
Raw dataset for Supplementary Fig. 29a,b.
Supplementary Table 41
Raw dataset for Supplementary Fig. 29c.
Supplementary Table 42
Raw dataset for Supplementary Fig. 29d.
Supplementary Table 43
Raw dataset for Supplementary Fig. 30a,b.
Supplementary Fig. 1
Unprocessed fluorescence images of blood samples for Supplementary Fig. 18a.
Supplementary PDF 1
Unprocessed fluorescence images of organs for Supplementary Fig. 19.
Supplementary PDF 2
Unprocessed fluorescence images of lower limb bones for Supplementary Fig. 20.
Supplementary PDF 3
Unprocessed HE staining of lungs for Supplementary Fig. 28a.
Supplementary PDF 4
Unprocessed immunohistochemical staining of CD8 + T cells in lung tissues for Supplementary Fig. 28c.
Supplementary PDF 5
Unprocessed immunohistochemical staining of CD8 + T cells in tumour tissues for Supplementary Fig. 29d.
Source data
Source Data Fig. 1
Unprocessed TEM and cryo-EM images of OMVs for Fig. 1a.
Source Data Fig. 4
Unprocessed immunofluorescence images and western blots for Fig. 4c,d.
Source Data Fig. 5
Unprocessed immunofluorescence images and western blots for Fig. 5c,d.
Source Data Fig. 1
Raw dataset for Fig. 1b,e–g.
Source Data Fig. 2
Raw dataset for Fig. 2a–c,e–g,i,k.
Source Data Fig. 3
Raw dataset for Fig. 3a,b,d,e,g–i.
Source Data Fig. 4
Raw dataset for Fig. 4b,c,e,g–j.
Source Data Fig. 5
Raw dataset for Fig. 5b,e,g–j.
Source Data Extended Data Fig. 1
Raw dataset for Extended Data Fig. 1a–b,d–f.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, G., Ma, N., Cheng, K. et al. Bacteria-derived nanovesicles enhance tumour vaccination by trained immunity. Nat. Nanotechnol. 19, 387–398 (2024). https://doi.org/10.1038/s41565-023-01553-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01553-6