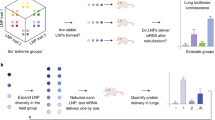
Abstract
Inhaled delivery of mRNA has the potential to treat a wide variety of diseases. However, nebulized mRNA lipid nanoparticles (LNPs) face several unique challenges including stability during nebulization and penetration through both cellular and extracellular barriers. Here we develop a combinatorial approach addressing these barriers. First, we observe that LNP formulations can be stabilized to resist nebulization-induced aggregation by altering the nebulization buffer to increase the LNP charge during nebulization, and by the addition of a branched polymeric excipient. Next, we synthesize a combinatorial library of ionizable, degradable lipids using reductive amination, and evaluate their delivery potential using fully differentiated air–liquid interface cultured primary lung epithelial cells. The final combination of ionizable lipid, charge-stabilized formulation and stability-enhancing excipient yields a significant improvement in lung mRNA delivery over current state-of-the-art LNPs and polymeric nanoparticles.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data that support the findings of this study are provided within the paper and its Supplementary Information. Source data are provided with this paper.
References
Kowalski, P. S., Rudra, A., Miao, L. & Anderson, D. G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol. Ther. 27, 710–728 (2019).
Hajj, K. A. & Whitehead, K. A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2, 1–17 (2017).
Han, X. et al. An ionizable lipid toolbox for RNA delivery. Nat. Commun. 12, 7233 (2021).
Qiu, M. et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc. Natl Acad. Sci. USA 118, e2020401118 (2021).
Swingle, K. L., Hamilton, A. G. & Mitchell, M. J. Lipid nanoparticle-mediated delivery of mRNA therapeutics and vaccines. Trends Mol. Med. 27, 616–617 (2021).
Miao, L. et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 37, 1174–1185 (2019).
Zhang, X. et al. Functionalized lipid-like nanoparticles for in vivo mRNA delivery and base editing. Sci. Adv. 6, eabc2315 (2020).
Billingsley, M. M. et al. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 20, 1578–1589 (2020).
Riley, R. S. et al. Ionizable lipid nanoparticles for in utero mRNA delivery. Sci. Adv. 7, eaba1028 (2021).
Sabnis, S. et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 26, 1509–1519 (2018).
Fenton, O. S. et al. Synthesis and biological evaluation of ionizable lipid materials for the in vivo delivery of messenger RNA to B lymphocytes. Adv. Mater. 29, 1606944 (2017).
Liu, J. et al. Fast and efficient CRISPR/Cas9 genome editing in vivo enabled by bioreducible lipid and messenger RNA nanoparticles. Adv. Mater. 31, 1902575 (2019).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Gillmore, J. D. et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385, 493–502 (2021).
Cornebise, M. et al. Discovery of a novel amino lipid that improves lipid nanoparticle performance through specific interactions with mRNA. Adv. Funct. Mater. https://doi.org/10.1002/adfm.202106727 (2021).
Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022).
Chakraborty, C., Sharma, A. R., Bhattacharya, M. & Lee, S.-S. From COVID-19 to cancer mRNA vaccines: moving from bench to clinic in the vaccine landscape. Front. Immunol. 12, 2648 (2021).
Cafri, G. et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J. Clin. Invest. 130, 5976–5988 (2020).
Oberli, M. A. et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 17, 1326–1335 (2017).
Espeseth, A. S. et al. Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection. NPJ Vaccines 5, 1–14 (2020).
Aliprantis, A. O. et al. A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion F protein vaccine in healthy younger and older adults. Hum. Vaccines Immunother. 17, 1248–1261 (2021).
Bahl, K. et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol. Ther. 25, 1316–1327 (2017).
Feldman, R. A. et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 37, 3326–3334 (2019).
John, S. et al. Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine 36, 1689–1699 (2018).
Medina-Magües, L. G. et al. mRNA vaccine protects against zika virus. Vaccines 9, 1464 (2021).
Mu, Z., Haynes, B. F. & Cain, D. W. HIV mRNA vaccines—progress and future paths. Vaccines 9, 134 (2021).
Zabaleta, N., Torella, L., Weber, N. D. & Gonzalez-Aseguinolaza, G. mRNA and gene editing: late breaking therapies in liver diseases. Hepatology https://doi.org/10.1002/hep.32441 (2022).
Robinson, E. et al. Lipid nanoparticle-delivered chemically modified mRNA restores chloride secretion in cystic fibrosis. Mol. Ther. 26, 2034–2046 (2018).
Da Silva Sanchez, A., Paunovska, K., Cristian, A. & Dahlman, J. E. Treating cystic fibrosis with mRNA and CRISPR. Hum. Gene Ther. 31, 940–955 (2020).
Lai, M. et al. Gene editing of DNAH11 restores normal cilia motility in primary ciliary dyskinesia. J. Med. Genet. 53, 242–249 (2016).
Paff, T., Omran, H., Nielsen, K. G. & Haarman, E. G. Current and future treatments in primary ciliary dyskinesia. Int. J. Mol. Sci. 22, 9834 (2021).
Guan, S., Darmstädter, M., Xu, C. & Rosenecker, J. In vitro investigations on optimizing and nebulization of IVT-mRNA formulations for potential pulmonary-based α-1-antitrypsin deficiency treatment. Pharmaceutics 13, 1281 (2021).
Zeyer, F. et al. mRNA-mediated gene supplementation of Toll-like receptors as treatment strategy for asthma in vivo. PLoS ONE 11, e0154001 (2016).
Mays, L. E. et al. Modified Foxp3 mRNA protects against asthma through an IL-10–dependent mechanism. J. Clin. Invest. 123, 1216–1228 (2013).
Rakhra, K. et al. Exploiting albumin as a mucosal vaccine chaperone for robust generation of lung-resident memory T cells. Sci. Immunol. 6, eabd8003 (2021).
Bivas-Benita, M. et al. Pulmonary delivery of chitosan-DNA nanoparticles enhances the immunogenicity of a DNA vaccine encoding HLA-A*0201-restricted T-cell epitopes of Mycobacterium tuberculosis. Vaccine 22, 1609–1615 (2004).
Rajapaksa, A. E. et al. Effective pulmonary delivery of an aerosolized plasmid DNA vaccine via surface acoustic wave nebulization. Respir. Res. 15, 60 (2014).
Wu, M. et al. Intranasal vaccination with mannosylated chitosan formulated DNA vaccine enables robust IgA and cellular response induction in the lungs of mice and improves protection against pulmonary mycobacterial challenge. Front. Cell. Infect. Microbiol. 7, 445 (2017).
King, R. G. et al. Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 and fully protects mice from lethal challenge. Vaccines 9, 881 (2021).
An, X. et al. Single-dose intranasal vaccination elicits systemic and mucosal immunity against SARS-CoV-2. iScience 24, 103037 (2021).
Kim, Y. C. et al. Strategy to enhance dendritic cell-mediated DNA vaccination in the lung. Adv. Ther. 3, 2000013 (2020).
Lu, D. & Hickey, A. J. Pulmonary vaccine delivery. Expert Rev. Vaccines 6, 213–226 (2007).
Sou, T. et al. New developments in dry powder pulmonary vaccine delivery. Trends Biotechnol. 29, 191–198 (2011).
Huang, J. et al. A novel dry powder influenza vaccine and intranasal delivery technology: induction of systemic and mucosal immune responses in rats. Vaccine 23, 794–801 (2004).
Minne, A. et al. The delivery site of a monovalent influenza vaccine within the respiratory tract impacts on the immune response. Immunology 122, 316–325 (2007).
Wang, Z. et al. Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat. Biomed. Eng. 6, 791–805 (2022).
Patel, A. K. et al. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv. Mater. 31, 1805116 (2019).
Lokugamage, M. P. et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat. Biomed. Eng. 5, 1059–1068 (2021).
Wilson, C. Future therapies for cystic fibrosis. Lancet Respir. Med. 10, e75–e76 (2022).
Witten, J., Samad, T. & Ribbeck, K. Selective permeability of mucus barriers. Curr. Opin. Biotechnol. 52, 124–133 (2018).
Witten, J. & Ribbeck, K. The particle in the spider’s web: transport through biological hydrogels. Nanoscale 9, 8080–8095 (2017).
Cone, R. A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 61, 75–85 (2009).
Lieleg, O. & Ribbeck, K. Biological hydrogels as selective diffusion barriers. Trends Cell Biol. 21, 543–551 (2011).
Kim, N., Duncan, G. A., Hanes, J. & Suk, J. S. Barriers to inhaled gene therapy of obstructive lung diseases: a review. J. Controlled Release 240, 465–488 (2016).
Coyne, C. B., Kelly, M. M., Boucher, R. C. & Johnson, L. G. Enhanced epithelial gene transfer by modulation of tight junctions with sodium caprate. Am. J. Respir. Cell Mol. Biol. 23, 602–609 (2000).
Kauffman, K. J. et al. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 15, 7300–7306 (2015).
Billingsley, M. M. et al. Orthogonal design of experiments for optimization of lipid nanoparticles for mRNA engineering of CAR T cells. Nano Lett. 22, 533–542 (2022).
Li, S. et al. Payload distribution and capacity of mRNA lipid nanoparticles. Nat. Commun. 13, 5561 (2022).
Kauffman, K. J. et al. Rapid, single-cell analysis and discovery of vectored mRNA transfection in vivo with a loxP-flanked tdTomato reporter mouse. Mol. Ther. Nucleic Acids 10, 55–63 (2018).
Ball, R. L., Bajaj, P. & Whitehead, K. A. Achieving long-term stability of lipid nanoparticles: examining the effect of pH, temperature, and lyophilization. Int. J. Nanomed. 12, 305–315 (2017).
Zhao, P. et al. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 5, 358–363 (2020).
Crowe, J. H., Oliver, A. E., Hoekstra, F. A. & Crowe, L. M. Stabilization of dry membranes by mixtures of hydroxyethyl starch and glucose: the role of vitrification. Cryobiology 35, 20–30 (1997).
Ohtake, S., Schebor, C., Palecek, S. P. & de Pablo, J. J. Phase behavior of freeze-dried phospholipid–cholesterol mixtures stabilized with trehalose. Biochim. Biophys. Acta Biomembr. 1713, 57–64 (2005).
Eastman, S. J. et al. Optimization of formulations and conditions for the aerosol delivery of functional cationic lipid:DNA complexes. Hum. Gene Ther. 8, 313–322 (1997).
Whitehead, K. A. et al. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat. Commun. 5, 4277 (2014).
Liu, S. et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR–Cas gene editing. Nat. Mater. 20, 701–710 (2021).
Pezzulo, A. A. et al. The air–liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L25–L31 (2011).
Hill, D. B. & Button, B. in Mucins: Methods and Protocols (eds McGuckin, M. A. & Thornton, D. J.) 245–258 (Humana Press, 2012); https://doi.org/10.1007/978-1-61779-513-8_15
Ramachandran, S. et al. Efficient delivery of RNA interference oligonucleotides to polarized airway epithelia in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L23–L32 (2013).
Krishnamurthy, S. et al. Manipulation of cell physiology enables gene silencing in well-differentiated airway epithelia. Mol. Ther. Nucleic Acids 1, e41 (2012).
Burgel, P.-R., Montani, D., Danel, C., Dusser, D. J. & Nadel, J. A. A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax 62, 153–161 (2007).
Ratjen, F. Cystic fibrosis: the role of the small airways. J. Aerosol Med. Pulm. Drug Deliv. 25, 261–264 (2012).
van den Berge, M., ten Hacken, N. H. T., Cohen, J., Douma, W. R. & Postma, D. S. Small airway disease in asthma and COPD: clinical implications. Chest 139, 412–423 (2011).
Tiddens, H. A. W. M., Donaldson, S. H., Rosenfeld, M. & Paré, P. D. Cystic fibrosis lung disease starts in the small airways: can we treat it more effectively? Pediatr. Pulmonol. 45, 107–117 (2010).
Tatsuta, M. et al. Effects of cigarette smoke on barrier function and tight junction proteins in the bronchial epithelium: protective role of cathelicidin LL-37. Respir. Res. 20, 251 (2019).
Maeki, M., Uno, S., Niwa, A., Okada, Y. & Tokeshi, M. Microfluidic technologies and devices for lipid nanoparticle-based RNA delivery. J. Control. Release 344, 80–96 (2022).
Cheng, M. H. Y. et al. Induction of bleb structures in lipid nanoparticle formulations of mRNA leads to improved transfection potency. Adv. Mater. https://doi.org/10.1002/adma.202303370 (2023).
Brader, M. L. et al. Encapsulation state of messenger RNA inside lipid nanoparticles. Biophys. J. 120, 2766–2770 (2021).
Kulkarni, J. A. et al. On the formation and morphology of lipid nanoparticles containing ionizable cationic lipids and siRNA. ACS Nano 12, 4787–4795 (2018).
Kulkarni, J. A. et al. Fusion-dependent formation of lipid nanoparticles containing macromolecular payloads. Nanoscale 11, 9023–9031 (2019).
Richardson, S. J., Bai, A., Kulkarni, A. A. & Moghaddam, M. F. Efficiency in drug discovery: liver S9 fraction assay as a screen for metabolic stability. Drug Metab. Lett. 10, 83–90 (2016).
Scholte, B. J., Davidson, D. J., Wilke, M. & de Jonge, H. R. Animal models of cystic fibrosis. J. Cyst. Fibros. 3, 183–190 (2004).
McCarron, A., Donnelley, M. & Parsons, D. Airway disease phenotypes in animal models of cystic fibrosis. Respir. Res. 19, 54 (2018).
Kim, N. et al. Inhaled gene therapy of preclinical muco-obstructive lung diseases by nanoparticles capable of breaching the airway mucus barrier. Thorax 77, 812–820 (2022).
Phillips, J. E., Zhang, X. & Johnston, J. A. Dry powder and nebulized aerosol inhalation of pharmaceuticals delivered to mice using a nose-only exposure system. J. Vis. Exp. https://doi.org/10.3791/55454 (2017).
Beck, S. E. et al. Deposition and expression of aerosolized rAAV vectors in the lungs of rhesus macaques. Mol. Ther. 6, 546–554 (2002).
Woo, C. J. et al. Inhaled delivery of a lipid nanoparticle encapsulated messenger RNA encoding a ciliary protein for the treatment of primary ciliary dyskinesia. Pulm. Pharmacol. Ther. 75, 102134 (2022).
Okuda, K. et al. Secretory cells dominate airway CFTR expression and function in human airway superficial epithelia. Am. J. Respir. Crit. Care Med. 203, 1275–1289 (2021).
Carraro, G. et al. Transcriptional analysis of cystic fibrosis airways at single-cell resolution reveals altered epithelial cell states and composition. Nat. Med. 27, 806–814 (2021).
Hodges, C. A. & Conlon, R. A. Delivering on the promise of gene editing for cystic fibrosis. Genes Dis. 6, 97–108 (2019).
Vanover, D. et al. Nebulized mRNA-encoded antibodies protect hamsters from SARS-CoV-2 infection. Adv. Sci. 9, 2202771 (2022).
Rhym, L. H., Manan, R. S., Koller, A., Stephanie, G. & Anderson, D. G. Peptide-encoding mRNA barcodes for the high-throughput in vivo screening of libraries of lipid nanoparticles for mRNA delivery. Nat. Biomed. Eng. 7, 901–910 (2023).
Chen, D. et al. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J. Am. Chem. Soc. 134, 6948–6951 (2012).
Heyes, J., Palmer, L., Bremner, K. & MacLachlan, I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J. Control. Release 107, 276–287 (2005).
Acknowledgements
This work was supported by the NIH (grant numbers UG3HL147367 and R01 HL162564-02 to A.Y.J., F.E., C.M. and D.G.A.) and Sanofi (formerly Translate Bio, to I.O.R., Y.H., R.S.M. and D.G.A.). J.W. was supported by the Cystic Fibrosis Foundation under award WITTEN19XX0. S.M. and F.A.O. were supported by the MIT Undergraduate Research Opportunities Program. We thank the Koch Institute Swanson Biotechnology Center for technical support, specifically the Animal Imaging & Preclinical Testing, Histology, Nanotechnology Materials, BioMicro Center and Microscopy core facilities. This work was also supported in part by the Koch Institute Support (core) Grant P30-CA14051 from the National Cancer Institute.
Author information
Authors and Affiliations
Contributions
A.Y.J., J.W. and I.O.R. conceived the project, contributed equally and all reserve the right to list themselves first on their CVs. A.Y.J., J.W., I.O.R., F.E., C.M., S.M., F.A.O., Y.H. and R.S.M. performed the experiments and analysed data. A.Y.J., J.W., R.L. and D.G.A. discussed the results and wrote the paper with input from all authors. R.L. and D.G.A. acquired funding and supervised the project.
Corresponding author
Ethics declarations
Competing interests
A.Y.J., J.W., I.O.R. and D.G.A. have filed a patent for the biodegradable lipid library described herein (US Patent Application No. 18080299). D.G.A. receives research funding from Sanofi/Translate Bio, and is a founder of oRNA Tx. R.L. is co-founder and a director of Moderna. He also serves on the board and has equity in Particles For Humanity. For a list of entities with which R.L. is, or has been recently involved, compensated or uncompensated, see https://www.dropbox.com/s/yc3xqb5s8s94v7x/Rev%20Langer%20COI.pdf?dl=0. The other authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Dan Peer, Bruno Pitard and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–23 and Table 1.
Supplementary Data 1
Statistical source data for the supplementary figures.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Fig. 4
Statistical source data for Fig. 4.
Source Data Fig. 5
Statistical source data for Fig. 5.
Source Data Fig. 6
Statistical source data for Fig. 6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, A.Y., Witten, J., Raji, I.O. et al. Combinatorial development of nebulized mRNA delivery formulations for the lungs. Nat. Nanotechnol. 19, 364–375 (2024). https://doi.org/10.1038/s41565-023-01548-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01548-3