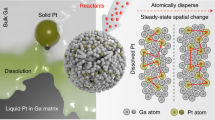
Abstract
The use of liquid gallium as a solvent for catalytic reactions has enabled access to well-dispersed metal atoms configurations, leading to unique catalytic phenomena, including activation of neighbouring liquid atoms and mobility-induced activity enhancement. To gain mechanistic insights into liquid metal catalysts, here we introduce a GaSn0.029Ni0.023 liquid alloy for selective propylene synthesis from decane. Owing to their mobility, dispersed atoms in a Ga matrix generate configurations where interfacial Sn and Ni atoms allow for critical alignments of reactants and intermediates. Computational modelling, corroborated by experimental analyses, suggests a particular reaction mechanism by which Sn protrudes from the interface and an adjacent Ni, below the interfacial layer, aligns precisely with a decane molecule, facilitating propylene production. We then apply this reaction pathway to canola oil, attaining a propylene selectivity of ~94.5%. Our results offer a mechanistic interpretation of liquid metal catalysts with an eye to potential practical applications of this technology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Kyriakou, G. et al. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. Science 335, 1209–1212 (2012).
Chen, S. et al. Propane dehydrogenation on single-site [PtZn4] intermetallic catalysts. Chem 7, 387–405 (2021).
Smit, B. & Maesen, T. L. M. Towards a molecular understanding of shape selectivity. Nature 451, 671–678 (2008).
Cai, W. et al. Subsurface catalysis-mediated selectivity of dehydrogenation reaction. Sci. Adv. 4, eaar5418 (2018).
Li, H. et al. Synergetic interaction between neighbouring platinum monomers in CO2 hydrogenation. Nat. Nanotechnol. 13, 411–417 (2018).
Greiner, M. T. et al. Free-atom-like d states in single-atom alloy catalysts. Nat. Chem. 10, 1008–1015 (2018).
Cui, T.-L. et al. Encapsulating palladium nanoparticles inside mesoporous MFI zeolite nanocrystals for shape-selective catalysis. Angew. Chem. Int. Ed. 55, 9178–9182 (2016).
Wang, C. et al. Fischer–Tropsch synthesis to olefins boosted by MFI zeolite nanosheets. Nat. Nanotechnol. 17, 714–720 (2022).
Liu, D., He, Q., Ding, S. & Song, L. Structural regulation and support coupling effect of single-atom catalysts for heterogeneous catalysis. Adv. Energy Mater. 10, 2001482 (2020).
Ma, T. et al. Toward phase and catalysis control: tracking the formation of intermetallic nanoparticles at atomic scale. Chem 5, 1235–1247 (2019).
Guo, W., Wang, Z., Wang, X. & Wu, Y. General design concept for single-atom catalysts toward heterogeneous catalysis. Adv. Mater. 33, 2004287 (2021).
Somorjai, G. A. & Park, J. Y. Molecular factors of catalytic selectivity. Angew. Chem. Int. Ed. 47, 9212–9228 (2008).
Rahim, M. A. et al. Low-temperature liquid platinum catalyst. Nat. Chem. 14, 935–941 (2022).
Zuraiqi, K. et al. Liquid metals in catalysis for energy applications. Joule 4, 2290–2321 (2020).
Yan, H. et al. Tandem In2O3-Pt/Al2O3 catalyst for coupling of propane dehydrogenation to selective H2 combustion. Science 371, 1257–1260 (2021).
Motagamwala, A. H., Almallahi, R., Wortman, J., Igenegbai, V. O. & Linic, S. Stable and selective catalysts for propane dehydrogenation operating at thermodynamic limit. Science 373, 217–222 (2021).
Tang, J. et al. Low temperature mechano-catalytic biofuel conversion using liquid metals. Chem. Eng. J. 452, 139350 (2023).
Liu, H. et al. Solid–liquid phase transition induced electrocatalytic switching from hydrogen evolution to highly selective CO2 reduction. Nat. Catal. 4, 202–211 (2021).
Studt, F. et al. Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nat. Chem. 6, 320–324 (2014).
Ma, Z. et al. Permeable superelastic liquid-metal fibre mat enables biocompatible and monolithic stretchable electronics. Nat. Mater. 20, 859–868 (2021).
Esrafilzadeh, D. et al. Room temperature CO2 reduction to solid carbon species on liquid metals featuring atomically thin ceria interfaces. Nat. Commun. 10, 865 (2019).
Tang, J. et al. Low temperature nano mechano-electrocatalytic CH4 conversion. ACS Nano 16, 8684–8693 (2022).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Tang, J. et al. Unique surface patterns emerging during solidification of liquid metal alloys. Nat. Nanotechnol. 16, 431–439 (2021).
Vanommeslaeghe, K. & MacKerell, A. D. Jr. Automation of the CHARMM General Force Field (CGenFF) I: bond perception and atom typing. J. Chem. Inf. Model. 52, 3144–3154 (2012).
Vanommeslaeghe, K., Raman, E. P. & MacKerell, A. D. Jr. Automation of the CHARMM General Force Field (CGenFF) II: assignment of bonded parameters and partial atomic charges. J. Chem. Inf. Model. 52, 3155–3168 (2012).
Martínez, L., Andrade, R., Birgin, E. G. & Martínez, J. M. PACKMOL: a package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 30, 2157–2164 (2009).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Tang, W., Sanville, E. & Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 21, 084204 (2009).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Giorgino, T. Computing diffusion coefficients in macromolecular simulations: the Diffusion Coefficient Tool for VMD. J. Open Source Softw. 4, 1698 (2019).
Acknowledgements
We thank D. Thomas of the Spectroscopy Laboratory and the Nuclear Magnetic Resonance Facility at the University of New South Wales, Sydney for his technical assistance. We also thank M. B. Ghasemian at the University of New South Wales, Sydney for his technical assistance. This work was supported by the Australian Research Council (ARC) Laureate Fellowship grant (FL180100053).
Author information
Authors and Affiliations
Contributions
Junma Tang initiated the concept and designed the experiments along with K.K.-Z. Junma Tang also conducted the experiments and carried out the characterizations, with the help of M.A.R. and K.K.-Z. The molecular dynamics simulations were performed by A.J.C., N.M. and S.P.R.; M.T. helped with the mass spectrometry experiments and analysis. P.V.K. and J.A.Y. performed the Bader charge analyses. The following individuals contributed to the data analyses, scientific discussions and authorship of the paper: A.J.C., J.S., M.T., N.M., Q.Z., Jianbo Tang, L.D., G.M., S.P.R., R.B.K., M.A.R. and K.K.-Z. All authors revised the manuscript and provided helpful comments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Shinya Furukawa, Yian Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussions 1–10, Figs. 1–22 and Tables 1–6.
Supplementary Video 1
The video shows the gaseous bubbles produced from the scaled-up experiment, using GaSn0.029Ni0.023 as the catalyst and canola oil as the feedstock.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, J., Christofferson, A.J., Sun, J. et al. Dynamic configurations of metallic atoms in the liquid state for selective propylene synthesis. Nat. Nanotechnol. 19, 306–310 (2024). https://doi.org/10.1038/s41565-023-01540-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01540-x