Abstract
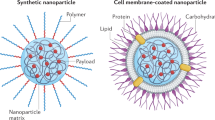
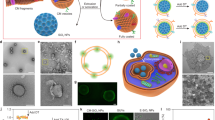
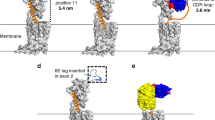
Since their initial development, cell membrane-coated nanoparticles (CNPs) have become increasingly popular in the biomedical field. Despite their inherent versatility and ability to enable complex biological applications, there is considerable interest in augmenting the performance of CNPs through the introduction of additional functionalities. Here we demonstrate a genetic-engineering-based modular approach to CNP functionalization that can encompass a wide range of ligands onto the nanoparticle surface. The cell membrane coating is engineered to express a SpyCatcher membrane anchor that can readily form a covalent bond with any moiety modified with SpyTag. To demonstrate the broad utility of this technique, three unique targeted CNP formulations are generated using different classes of targeting ligands, including a designed ankyrin repeat protein, an affibody and a single-chain variable fragment. In vitro, the modified nanoparticles exhibit enhanced affinity towards cell lines overexpressing the cognate receptors for each ligand. When formulated with a chemotherapeutic payload, the modularly functionalized nanoparticles display strong targeting ability and growth suppression in a murine tumour xenograft model of ovarian cancer. Our data suggest genetic engineering offers a feasible approach for accelerating the development of multifunctional CNPs for a broad range of biomedical applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within this article and its Supplementary Information. Source data are provided with this paper.
References
Fang, R. H., Kroll, A. V., Gao, W. & Zhang, L. Cell membrane coating nanotechnology. Adv. Mater. 30, 1706759 (2018).
Fang, R. H., Gao, W. & Zhang, L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol. 20, 33–48 (2023).
Narain, A., Asawa, S., Chhabria, V. & Patil-Sen, Y. Cell membrane coated nanoparticles: next-generation therapeutics. Nanomedicine 12, 2677–2692 (2017).
Hu, C. M. et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl Acad. Sci. USA 108, 10980–10985 (2011).
Fang, R. H. et al. Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles. Nanoscale 5, 8884–8888 (2013).
Liu, G. et al. Engineering biomimetic platesomes for pH-responsive drug delivery and enhanced antitumor activity. Adv. Mater. 31, 1900795 (2019).
Hu, Q. et al. Anticancer platelet-mimicking nanovehicles. Adv. Mater. 27, 7043–7050 (2015).
Chen, H. et al. Lipid insertion enables targeted functionalization of paclitaxel-loaded erythrocyte membrane nanosystem by tumor-penetrating bispecific recombinant protein. Int. J. Nanomed. 13, 5347–5359 (2018).
Li, P. Y., Fan, Z. & Cheng, H. Cell membrane bioconjugation and membrane-derived nanomaterials for immunotherapy. Bioconjug. Chem. 29, 624–634 (2018).
Fu, Q. et al. Programmed co-delivery of paclitaxel and doxorubicin boosted by camouflaging with erythrocyte membrane. Nanoscale 7, 4020–4030 (2015).
Zhu, D. M. et al. Erythrocyte membrane-coated gold nanocages for targeted photothermal and chemical cancer therapy. Nanotechnology 29, 084002 (2018).
Zhang, Q. et al. Biomimetic magnetosomes as versatile artificial antigen-presenting cells to potentiate T-cell-based anticancer therapy. ACS Nano 11, 10724–10732 (2017).
Han, Y. et al. T cell membrane mimicking nanoparticles with bioorthogonal targeting and immune recognition for enhanced photothermal therapy. Adv. Sci. 6, 1900251 (2019).
Ma, W. et al. Coating biomimetic nanoparticles with chimeric antigen receptor T cell-membrane provides high specificity for hepatocellular carcinoma photothermal therapy treatment. Theranostics 10, 1281–1295 (2020).
Rao, L. et al. Activating macrophage-mediated cancer immunotherapy by genetically edited nanoparticles. Adv. Mater. 32, 2004853 (2020).
Zhang, X. et al. PD-1 blockade cellular vesicles for cancer immunotherapy. Adv. Mater. 30, 1707112 (2018).
Jiang, Y. et al. Engineered cell-membrane-coated nanoparticles directly present tumor antigens to promote anticancer immunity. Adv. Mater. 32, 2001808 (2020).
Bose, R. J. et al. Bioengineered stem cell membrane functionalized nanocarriers for therapeutic targeting of severe hindlimb ischemia. Biomaterials 185, 360–370 (2018).
Park, J. H. et al. Virus-mimicking cell membrane-coated nanoparticles for cytosolic delivery of mRNA. Angew. Chem. Int. Ed. 61, e202113671 (2022).
Saeui, C. T., Mathew, M. P., Liu, L., Urias, E. & Yarema, K. J. Cell surface and membrane engineering: emerging technologies and applications. J. Funct. Biomater. 6, 454–485 (2015).
Yu, K., Liu, C., Kim, B. G. & Lee, D. Y. Synthetic fusion protein design and applications. Biotechnol. Adv. 33, 155–164 (2015).
Cho, J. H., Collins, J. J. & Wong, W. W. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell 173, 1426–1438.e11 (2018).
van der Meer, S. B. et al. Avidin-conjugated calcium phosphate nanoparticles as a modular targeting system for the attachment of biotinylated molecules in vitro and in vivo. Acta Biomater. 57, 414–425 (2017).
Peuler, K., Dimmitt, N. & Lin, C. C. Clickable modular polysaccharide nanoparticles for selective cell-targeting. Carbohydr. Polym. 234, 115901 (2020).
Vragniau, C. et al. Synthetic self-assembling ADDomer platform for highly efficient vaccination by genetically encoded multiepitope display. Sci. Adv. 5, eaaw2853 (2019).
Brouwer, P. J. M. et al. Two-component spike nanoparticle vaccine protects macaques from SARS-CoV-2 infection. Cell 184, 1188–1200.e19 (2021).
Li, X. et al. Orthogonal modular biosynthesis of nanoscale conjugate vaccines for vaccination against infection. Nano Res. 15, 1645–1653 (2022).
Bruun, T. U. J., Andersson, A. C., Draper, S. J. & Howarth, M. Engineering a rugged nanoscaffold to enhance plug-and-display vaccination. ACS Nano 12, 8855–8866 (2018).
Singh, S. K. et al. Improving the malaria transmission-blocking activity of a Plasmodium falciparum 48/45 based vaccine antigen by SpyTag/SpyCatcher mediated virus-like display. Vaccine 35, 3726–3732 (2017).
Wang, W. et al. Ferritin nanoparticle-based SpyTag/SpyCatcher-enabled click vaccine for tumor immunotherapy. Nanomedicine 16, 69–78 (2019).
Keeble, A. H. et al. Approaching infinite affinity through engineering of peptide–protein interaction. Proc. Natl Acad. Sci. USA 116, 26523–26533 (2019).
Pruszynski, M., D’Huyvetter, M., Bruchertseifer, F., Morgenstern, A. & Lahoutte, T. Evaluation of an anti-HER2 nanobody labeled with 225Ac for targeted α-particle therapy of cancer. Mol. Pharm. 15, 1457–1466 (2018).
Subik, K. et al. The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer Basic Clin. Res. 4, 35–41 (2010).
Wang, K., Li, D. & Sun, L. High levels of EGFR expression in tumor stroma are associated with aggressive clinical features in epithelial ovarian cancer. OncoTargets Ther. 9, 377–386 (2016).
Luk, B. T. et al. Interfacial interactions between natural RBC membranes and synthetic polymeric nanoparticles. Nanoscale 6, 2730–2737 (2014).
Hu, C. M. et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526, 118–121 (2015).
Hu, C. M. et al. ‘Marker-of-self’ functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale 5, 2664–2668 (2013).
Park, J. H. et al. Genetically engineered cell membrane-coated nanoparticles for targeted delivery of dexamethasone to inflamed lungs. Sci. Adv. 7, eabf7820 (2021).
Veggiani, G. et al. Programmable polyproteams built using twin peptide superglues. Proc. Natl Acad. Sci. USA 113, 1202–1207 (2016).
Chabloz, A. et al. Salmonella-based platform for efficient delivery of functional binding proteins to the cytosol. Commun. Biol. 3, 342 (2020).
Wang, Y. et al. Fluorogen activating protein-affibody probes: modular, no-wash measurement of epidermal growth factor receptors. Bioconjug. Chem. 26, 137–144 (2015).
Kroll, A. V. et al. Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity. Adv. Mater. 29, 1703969 (2017).
Acknowledgements
This work is supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense under grant number HDTRA1‐21‐1‐0010, the National Institutes of Health under award numbers R01CA200574, R21AI175904 and R21AI159492, and the National Science Foundation Grant DMR-1904702.
Author information
Authors and Affiliations
Contributions
N.K., Y.J., R.H.F. and L.Z. conceived and designed the experiments. N.K. conducted all experiments. Y.J., Y.D. and S.C. helped with plasmid construction and cell engineering. Y.J. and F.-X.P. assisted with protein production, cell culture and in vitro assays. J.Z. helped with all in vivo studies. A.M. supported the biodistribution and safety studies. M.H. and Z.G. assisted with tumour efficacy studies. N.K., Y.J., J.Z., A.M., F.-X.P., Y.D., M.H., S.C., Z.G., W.G., R.H.F. and L.Z. analysed and discussed the data. N.K., R.H.F. and L.Z. interpreted the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Mónica Fanarraga, Vesa-Pekka Lehto and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–31.
Supplementary Data
Statistical source data for all supplementary figures.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krishnan, N., Jiang, Y., Zhou, J. et al. A modular approach to enhancing cell membrane-coated nanoparticle functionality using genetic engineering. Nat. Nanotechnol. 19, 345–353 (2024). https://doi.org/10.1038/s41565-023-01533-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01533-w