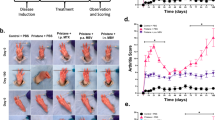
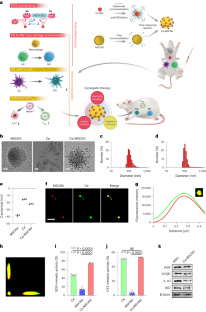
Abstract
Commencing with the breakdown of immune tolerance, multiple pathogenic factors, including synovial inflammation and harmful cytokines, are conjointly involved in the progression of rheumatoid arthritis. Intervening to mitigate some of these factors can bring a short-term therapeutic effect, but other unresolved factors will continue to aggravate the disease. Here we developed a ceria nanoparticle-immobilized mesenchymal stem cell nanovesicle hybrid system to address multiple factors in rheumatoid arthritis. Each component of this nanohybrid works individually and also synergistically, resulting in comprehensive treatment. Alleviation of inflammation and modulation of the tissue environment into an immunotolerant-favourable state are combined to recover the immune system by bridging innate and adaptive immunity. The therapy is shown to successfully treat and prevent rheumatoid arthritis by relieving the main symptoms and also by restoring the immune system through the induction of regulatory T cells in a mouse model of collagen-induced arthritis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper and the Supplementary Information. Source data are available for Figs. 1–6 and Supplementary Figs. 1, 3, 5–16, 20–23, 25–33, 35 and 36 in the associated source data file. Additional materials from this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
Change history
17 November 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41565-023-01568-z
References
Firestein, G. S. Evolving concepts of rheumatoid arthritis. Nature 423, 356–361 (2003).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Burmester, G. R., Feist, E. & Dörner, T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat. Rev. Rheumatol. 10, 77–88 (2014).
Van Vollenhoven, R. F. Treatment of rheumatoid arthritis: state of the art. Nat. Rev. Rheumatol. 5, 531–541 (2009).
Aletaha, D. & Smolen, J. S. Diagnosis and management of rheumatoid arthritis: a review. JAMA 320, 1360–1372 (2018).
Zhu, Y. et al. Rheumatoid arthritis microenvironment insights into treatment effect of nanomaterials. Nano Today 42, 101358 (2022).
Esensten, J. H., Wofsy, D. & Bluestone, J. A. Regulatory T cells as therapeutic targets in rheumatoid arthritis. Nat. Rev. Rheumatol. 5, 560–565 (2009).
Kim, J. et al. Synergistic oxygen generation and reactive oxygen species scavenging by manganese ferrite/ceria Co-decorated nanoparticles for rheumatoid arthritis treatment. ACS Nano 13, 3206–3217 (2019).
Zhu, L. et al. TSC1 controls macrophage polarization to prevent inflammatory disease. Nat. Commun. 5, 4696 (2014).
Weyand, C. M. & Goronzy, J. J. Immunometabolism in early and late stages of rheumatoid arthritis. Nat. Rev. Immunol. 13, 291–301 (2017).
Yang, C. et al. Inorganic nanosheets facilitate humoral immunity against medical implant infections by modulating immune co-stimulatory pathways. Nat. Commun. 13, 4866 (2022).
Wu, W. et al. Microbiotic nanomedicine for tumor-specific chemotherapy-synergized innate/adaptive antitumor immunity. Nano Today 42, 101377 (2022).
McInnes, I. B. & Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 7, 429–442 (2007).
Pelaz, B. et al. Diverse applications of nanomedicine. ACS Nano 11, 2313–2381 (2017).
Dominguez-Villar, M. & Hafler, D. A. Regulatory T cells in autoimmune disease. Nat. Immunol. 19, 665–673 (2018).
Komatsu, N. et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 20, 62–68 (2014).
Kim, C. K. et al. Ceria nanoparticles that can protect against ischemic stroke. Angew. Chem. Int. Ed. 51, 11039–11043 (2012).
Soh, M. et al. Ceria–zirconia nanoparticles as an enhanced multi-antioxidant for sepsis treatment. Angew. Chem. Int. Ed. 56, 11399–11403 (2017).
Nguyen, L., Bang, S. & Noh, I. Tissue regeneration of human mesenchymal stem cells on porous gelatin micro-carriers by long-term dynamic in vitro culture. Tissue Eng. Regen. Med. 16, 19–28 (2019).
Jiang, W. & Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 53, e12712 (2022).
Suryaprakash, S. et al. Engineered mesenchymal stem cell/nanomedicine spheroid as an active drug delivery platform for combinational glioblastoma therapy. Nano Lett. 19, 1701–1705 (2019).
Lu, K. et al. Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nat. Biomed. Eng. 2, 600–610 (2018).
Shahir, M. et al. Effect of mesenchymal stem cell‐derived exosomes on the induction of mouse tolerogenic dendritic cells. J. Cell. Physiol. 235, 7043–7055 (2020).
Gao, J., Gu, H. & Xu, B. Multifunctional magnetic nanoparticles: design, synthesis, and biomedical applications. Acc. Chem. Res. 42, 1097–1107 (2009).
Pelaz, B. et al. Surface functionalization of nanoparticles with polyethylene glycol: effects on protein adsorption and cellular uptake. ACS Nano 9, 6996–7008 (2015).
Koo, S. et al. Enhanced chemodynamic therapy by Cu–Fe peroxide nanoparticles: tumor microenvironment-mediated synergistic Fenton reaction. ACS Nano 16, 2535–2545 (2022).
Mittal, M. et al. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 20, 1126–1167 (2014).
Kemp, K. Mesenchymal stem cell‐secreted superoxide dismutase promotes cerebellar neuronal survival. J. Neurochem. 114, 1569–1580 (2010).
Uccelli, A., Moretta, L. & Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8, 726–736 (2008).
Adams, D. O. & Hamilton, T. A. The cell biology of macrophage activation. Annu. Rev. Immunol. 2, 283–318 (1984).
Richard, M. P. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat. Rev. Immunol. 2, 527–535 (2002).
Cifuentes-Rius, A. et al. Inducing immune tolerance with dendritic cell-targeting nanomedicines. Nat. Nanotechnol. 16, 37–46 (2021).
Hilkens, C. & Isaacs, J. Tolerogenic dendritic cell therapy for rheumatoid arthritis: where are we now? Clin. Exp. Immunol. 172, 148–157 (2013).
Zhang, B. et al. Site-specific PEGylation of interleukin-2 enhances immunosuppression via the sustained activation of regulatory T cells. Nat. Biomed. Eng. 5, 1288–1305 (2021).
Peng, B. et al. Tuned cationic dendronized polymer: molecular scavenger for rheumatoid arthritis treatment. Angew. Chem. Int. Ed. 58, 4254–4258 (2019).
Inglis, J. J. et al. Collagen‐induced arthritis as a model of hyperalgesia: functional and cellular analysis of the analgesic actions of tumor necrosis factor blockade. Arthritis Rheumatol. 56, 4015–4023 (2007).
Ruiz-Fernández, C. et al. WISP-2 modulates the induction of inflammatory mediators and cartilage catabolism in chondrocytes. Lab. Invest. 102, 989–999 (2022).
Barbi, J. et al. Metabolic control of the Treg/Th17 axis. Immunol. Rev. 252, 52–77 (2013).
Groux, H. et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389, 737–742 (1997).
Desreumaux, P. et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology 143, 1207–1217 (2012).
Reife, R. A. et al. SWR mice are resistant to collagen-induced arthritis but produce potentially arthritogenic antibodies. Arthritis Rheumatol. 34, 776–781 (1991).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Korn, T. et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007).
Sakaguchi, S. et al. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Dobrovolskaia, M. A. & McNeil, S. E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2, 469–478 (2007).
Hoshyar, N., Gray, S., Han, H. & Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 11, 673–692 (2016).
Madaan, A. et al. A stepwise procedure for isolation of murine bone marrow and generation of dendritic cells. J. Biol. Methods 1, 1–6 (2014).
Brand, D. D., Latham, K. A. & Rosloniec, E. F. Collagen-induced arthritis. Nat. Protoc. 2, 1269–1275 (2007).
Gao, X. H. et al. A store-operated calcium channel inhibitor attenuates collagen-induced arthritis. Br. J. Pharmacol. 172, 2991–3002 (2015).
Schmitz, N., Laverty, S., Kraus, V. B. & Aigner, T. Basic methods in histopathology of joint tissues. Osteoarthr. Cartil. 18, 113–116 (2010).
Kim, J. E. et al. Effect of self-assembled peptide-mesenchymal stem cell complex on the progression of osteoarthritis in a rat model. Int. J. Nanomed. 9, 141–157 (2014).
Acknowledgements
This work was supported by the Institute for Basic Science (IBS-R006-D1) (S.K. and T.H.), a Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korean government (the Ministry of Science and ICT, the Ministry of Health & Welfare) (21A0504L1-11) (T.H.K., S.Y.J., S.Y., J.Y. and Y.J), and a National Research Foundation of Korea (NRF) grant funded by the Korean government (2019M3A9H1103651) (H.S.S., C.K. and B.-S.K.).
Author information
Authors and Affiliations
Contributions
S.K., H.S.S. and T.H.K. conceived and designed the project. S.K., H.S.S., T.H.K., S. Yang, S.Y.J., S. Ye. and S.H.K. performed the experiments and analysed the data. B.C., K.S.P., H.M.S., O.K.P., C.K., M.K., M.S., J.Y., D.K. and N.L. provided technical input on this project. S.K., H.S.S., T.H.K., D.K., B.-S.K., Y.J. and T.H. wrote the manuscript. B.-S.K., Y.J. and T.H. supervised the overall research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Liangfang Zhang, Mauro Perretti, and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion, Figs. 1–36, unprocessed gel images for supplementary figures, gating strategy for flow cytometry, and references.
Supplementary data 1
Sequences of primers for PCR analysis.
Supplementary data 2
Source data for Supplementary Figures
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 5
Statistical Source Data
Source Data Fig. 6
Statistical Source Data
Source Data Fig. 1k
Unprocessed western blots
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koo, S., Sohn, H.S., Kim, T.H. et al. Ceria-vesicle nanohybrid therapeutic for modulation of innate and adaptive immunity in a collagen-induced arthritis model. Nat. Nanotechnol. 18, 1502–1514 (2023). https://doi.org/10.1038/s41565-023-01523-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01523-y
This article is cited by
-
Nanohybrid therapy hits multiple arthritis targets
Nature Reviews Rheumatology (2024)
-
Biohybrid nanoparticles for treating arthritis
Nature Nanotechnology (2023)