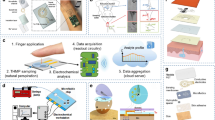
Abstract
Personalized monitoring of female hormones (for example, oestradiol) is of great interest in fertility and women’s health. However, existing approaches usually require invasive blood draws and/or bulky analytical laboratory equipment, making them hard to implement at home. Here we report a skin-interfaced wearable aptamer nanobiosensor based on target-induced strand displacement for automatic and non-invasive monitoring of oestradiol via in situ sweat analysis. The reagentless, amplification-free and ‘signal-on’ detection approach coupled with a gold nanoparticle-MXene-based detection electrode offers extraordinary sensitivity with an ultra-low limit of detection of 0.14 pM. This fully integrated system is capable of autonomous sweat induction at rest via iontophoresis, precise microfluidic sweat sampling controlled via capillary bursting valves, real-time oestradiol analysis and calibration with simultaneously collected multivariate information (that is, temperature, pH and ionic strength), as well as signal processing and wireless communication with a user interface (for example, smartphone). We validated the technology in human participants. Our data indicate a cyclical fluctuation in sweat oestradiol during menstrual cycles, and a high correlation between sweat and blood oestradiol was identified. Our study opens up the potential for wearable sensors for non-invasive, personalized reproductive hormone monitoring.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shansky, R. M. Are hormones a ‘female problem’ for animal research? Science 364, 825–826 (2019).
Albert, P. R. Why is depression more prevalent in women? J. Psychiatry Neurosci. 40, 219–221 (2015).
Nett, T. M., Turzillo, A. M., Baratta, M. & Rispoli, L. A. Pituitary effects of steroid hormones on secretion of follicle-stimulating hormone and luteinizing hormone. Domest. Anim. Endocrinol. 23, 33–42 (2002).
Luine, V. N. Estradiol and cognitive function: past, present and future. Horm. Behav. 66, 602–618 (2014).
van den Beld, A. W., de Jong, F. H., Grobbee, D. E., Pols, H. A. P. & Lamberts, S. W. J. Measures of bioavailable derum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J. Clin. Endocr. 85, 3276–3282 (2000).
Knowlton, A. A. & Lee, A. R. Estrogen and the cardiovascular system. Pharmacol. Ther. 135, 54–70 (2012).
Khosla, S., Oursler, M. J. & Monroe, D. G. Estrogen and the skeleton. Trends Endocrinol. Metab. 23, 576–581 (2012).
Rosner, W., Hankinson, S. E., Sluss, P. M., Vesper, H. W. & Wierman, M. E. Challenges to the measurement of estradiol: an endocrine society position statement. J. Clin. Endocrinol. Metab. 98, 1376–1387 (2013).
Karashima, S. & Osaka, I. Rapidity and precision of steroid hormone measurement. J. Clin. Med. 11, 956 (2022).
Macsali, F. et al. Menstrual cycle and respiratory symptoms in a general Nordic–Baltic population. Am. J. Respir. Crit. Care Med. 187, 366–373 (2013).
Rizk, B. & Smitz, J. Ovarian hyperstimulation syndrome after superovulation using GnRH agonists for IVF and related procedures. Hum. Reprod. 7, 320–327 (1992).
Klaiber, E. L., Broverman, D. M., Vogel, W., Peterson, L. G. & Snyder, M. B. Relationships of serum estradiol levels, menopausal duration, and mood during hormonal replacement therapy. Psychoneuroendocrinology 22, 549–558 (1997).
Geraci, A. et al. Sarcopenia and menopause: the role of estradiol. Front. Endocrinol. 12, 682012 (2021).
Dey, S. et al. Cyclic regulation of sensory perception by a female hormone alters behavior. Cell 161, 1334–1344 (2015).
Thienpont, L. M., Verhaeghe, P. G., Van Brussel, K. A. & De Leenheer, A. P. Estradiol-17 beta quantified in serum by isotope dilution-gas chromatography-mass spectrometry: reversed-phase C18 high-performance liquid chromatography compared with immuno-affinity chromatography for sample pretreatment. Clin. Chem. 34, 2066–2069 (1988).
Stanczyk, F. Z., Jurow, J. & Hsing, A. W. Limitations of direct immunoassays for measuring circulating estradiol levels in postmenopausal women and men in epidemiologic studies. Cancer Epidemiol. Biomark. Prev. 19, 903–906 (2010).
Lee, J. S. et al. Comparison of methods to measure low serum estradiol levels in postmenopausal women. J. Clin. Endocrinol. Metab. 91, 3791–3797 (2006).
Ettinger, B. et al. Associations between low levels of serum estradiol, bone density, and fractures among elderly women: the study of osteoporotic fractures. J. Clin. Endocrinol. Metab. 83, 2239–2243 (1998).
Ankarberg-Lindgren, C. & Norjavaara, E. A purification step prior to commercial sensitive immunoassay is necessary to achieve clinical usefulness when quantifying serum 17beta-estradiol in prepubertal children. Eur. J. Endocrinol. 158, 117–124 (2008).
Seippel, L. & Bäckström, T. Luteal-phase estradiol relates to symptom severity in patients with premenstrual syndrome. J. Clin. Endocr. Metab. 83, 1988–1992 (1998).
Yang, D. S., Ghaffari, R. & Rogers, J. A. Sweat as a diagnostic biofluid. Science 379, 760–761 (2023).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Lee, H. et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 11, 566–572 (2016).
Yang, Y. et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 38, 217–224 (2020).
Sempionatto, J. R. et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 5, 737–748 (2021).
Ates, H. C. et al. End-to-end design of wearable sensors. Nat. Rev. Mater. 7, 887–907 (2022).
Yang, Y. & Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 48, 1465–1491 (2019).
Sempionatto, J. R., Lasalde-Ramírez, J. A., Mahato, K., Wang, J. & Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 6, 899–915 (2022).
Kim, J., Campbell, A. S., de Ávila, B. E.-F. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019).
Heikenfeld, J. et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 37, 407–419 (2019).
Sinha, A. et al. MXene: an emerging material for sensing and biosensing. Trends Anal. Chem. 105, 424–435 (2018).
Babar, Z. U. D., Della Ventura, B., Velotta, R. & Iannotti, V. Advances and emerging challenges in MXenes and their nanocomposites for biosensing applications. RSC Adv. 12, 19590–19610 (2022).
UNAFold Web Server. DNA Folding Form (n.d.); http://www.unafold.org/mfold/applications/dna-folding-form.php
Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 (2003).
Rangel, A. E., Hariri, A. A., Eisenstein, M. & Soh, H. T. Engineering aptamer switches for multifunctional stimulus‐responsive nanosystems. Adv. Mater. 32, 2003704 (2020).
Pandey, R. et al. Integrating programmable DNAzymes with electrical readout for rapid and culture-free bacterial detection using a handheld platform. Nat. Chem. 13, 895–901 (2021).
Li, J., Rossignol, F. & Macdonald, J. Inkjet printing for biosensor fabrication: combining chemistry and technology for advanced manufacturing. Lab. Chip 15, 2538–2558 (2015).
VahidMohammadi, A., Rosen, J. & Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 372, eabf1581 (2021).
Wang, M. et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6, 1225–1235 (2022).
Yu, Y. et al. Biofuel-powered soft electronic skin with multiplexed and wireless sensing for human-machine interfaces. Sci. Robot. 5, eaaz7946 (2020).
Min, J. et al. An autonomous wearable biosensor powered by a perovskite solar cell. Nat. Electron. 6, 630–641 (2023).
Choi, J., Kang, D., Han, S., Kim, S. B. & Rogers, J. A. Thin, soft, skin-mounted microfluidic networks with capillary bursting valves for chrono-sampling of sweat. Adv. Healthc. Mater. 6, 1601355 (2017).
Chenault, J. R., Thatcher, W. W., Kalra, P. S., Abrams, R. M. & Wilcox, C. J. Transitory changes in plasma progestins, estradiol, and luteinizing hormone approaching ovulation in the bovine. J. Dairy Sc. 58, 709–717 (1975).
Alhabeb, M. et al. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 29, 7633–7644 (2017).
Torrente-Rodríguez, R. M. et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2, 921–937 (2020).
Capatina, D. et al. Label-free electrochemical aptasensor for the detection of the 3-O-C12-HSL quorum-sensing molecule in Pseudomonas aeruginosa. Biosensors 12, 440 (2022).
Torrente-Rodríguez, R. M. et al. SARS-CoV-2 RapidPlex: a graphene-based multiplexed telemedicine platform for rapid and low-cost COVID-19 diagnosis and monitoring. Matter 3, 1981–1998 (2020).
Frum, Y., Bonner, M. C., Eccleston, G. M. & Meidan, V. M. The influence of drug partition coefficient on follicular penetration: In vitro human skin studies. Eur. J. Pharm. Sci. 30, 280–287 (2007).
Sparrenberg, L. T., Greiner, B. & Mathis, H. P. Bleaching correction for DNA measurements in highly diluted solutions using confocal microscopy. PLoS ONE 15, e0231918 (2020).
Salieb-Beugelaar, G. B., Dorfman, K. D., van den Berg, A. & Eijkel, J. C. T. Electrophoretic separation of DNA in gels and nanostructures. Lab. Chip 9, 2508 (2009).
Acknowledgements
This project was supported by the National Institutes of Health grant nos. R01HL155815 and R21DK13266, National Science Foundation grant no. 2145802, Office of Naval Research grant nos. N00014-21-1-2483 and N00014-21-1-2845, American Cancer Society Research Scholar grant no. RSG-21-181-01-CTPS and a Sloan Research Fellowship. We gratefully acknowledge critical support and infrastructure provided for this work by the Kavli Nanoscience Institute at Caltech.
Author information
Authors and Affiliations
Contributions
W.G. and C.Y. initiated the concept and designed the overall studies. W.G. supervised the work. C.Y. and M.W. led the experiments and collected the overall data. J.M., R.Y.T., H.L., J.R.S. and C.X. contributed to sensor characterization and validation. J.L. contributed to the numerical simulation. All authors contributed the data analysis and provided feedback on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Fabiana Arduini, Eden Morales-Narvaez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, discussion, figures, tables, references and video captions.
Supplementary Video 1
Numerical simulation of the electric field enhanced oestradiol sensing.
Supplementary Video 2
Microfluidic sweat sampling using a finger-worn wearable sensor patch.
Supplementary Video 3
Real-time wireless oestradiol monitoring.
Source data
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, C., Wang, M., Min, J. et al. A wearable aptamer nanobiosensor for non-invasive female hormone monitoring. Nat. Nanotechnol. 19, 330–337 (2024). https://doi.org/10.1038/s41565-023-01513-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01513-0
This article is cited by
-
Interstitial fluid-based wearable biosensors for minimally invasive healthcare and biomedical applications
Communications Materials (2024)
-
Advancing the neuroscience of human pregnancy
Nature Neuroscience (2024)
-
Wireless real-time monitoring of oestradiol in sweat
Nature Nanotechnology (2024)
-
The potential of wearable sweat sensors in heart failure management
Nature Electronics (2024)
-
Long-term monitoring of ultratrace nucleic acids using tetrahedral nanostructure-based NgAgo on wearable microneedles
Nature Communications (2024)