Abstract
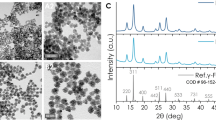
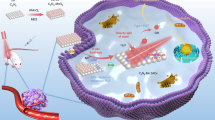
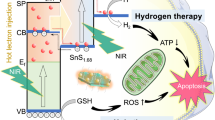
Dynamic therapies have potential in cancer treatments but have limitations in efficiency and penetration depth. Here a membrane-integrated liposome (MIL) is created to coat titanium dioxide (TiO2) nanoparticles to enhance electron transfer and increase radical production under low-dose X-ray irradiation. The exoelectrogenic Shewanella oneidensis MR-1 microorganism presents an innate capability for extracellular electron transfer (EET). An EET-mimicking photocatalytic system is created by coating the TiO2 nanoparticles with the MIL, which significantly enhances superoxide anions generation under low-dose (1 Gy) X-ray activation. The c-type cytochromes-constructed electron channel in the membrane mimics electron transfer to surrounding oxygen. Moreover, the hole transport in the valence band is also observed for water oxidation to produce hydroxyl radicals. The TiO2@MIL system is demonstrated against orthotopic liver tumours in vivo.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Shi, L. et al. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 14, 651–662 (2016).
Silhavy, T. J., Kahne, D. & Walker, S. The bacterial cell envelope. CSH Perspect. Biol. 2, a000414 (2010).
Castelle, C. et al. A new iron-oxidizing/O2-reducing supercomplex spanning both inner and outer membranes, isolated from the extreme acidophile Acidithiobacillus ferrooxidans. J. Biol. Chem. 283, 25803–25811 (2008).
Beliaev, A. S., Saffarini, D. A., McLaughlin, J. L. & Hunnicutt, D. MtrC, an outer membrane decahaem c cytochrome required for metal reduction in Shewanella putrefaciens MR-1. Mol. Microbiol. 39, 722–730 (2001).
Myers, C. R. & Myers, J. M. MtrB is required for proper incorporation of the cytochromes OmcA and OmcB into the outer membrane of Shewanella putrefaciens MR-1. Appl. Environ. Microb. 68, 5585–5594 (2002).
Bretschger, O. et al. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl. Environ. Microbiol. 73, 7003–7012 (2007).
Edwards, M. J., White, G. F., Butt, J. N., Richardson, D. J. & Clarke, T. A. The crystal structure of a biological insulated transmembrane molecular wire. Cell 181, 665–673 (2020).
Choi, S. Electrogenic bacteria promise new opportunities for powering, sensing, and synthesizing. Small 18, 2107902 (2022).
Ainsworth, E. V. et al. Photoreduction of Shewanella oneidensis extracellular cytochromes by organic chromophores and dye-sensitized TiO2. ChemBioChem 17, 2324–2333 (2016).
Liu, L. & Choi, S. Enhanced biophotoelectricity generation in cyanobacterial biophotovoltaics with intracellularly biosynthesized gold nanoparticles. J. Power Sources 506, 230251 (2021).
Deng, X., Luo, D. & Okamoto, A. Defined and unknown roles of conductive nanoparticles for the enhancement of microbial current generation: a review. Bioresour. Technol. 350, 126844 (2022).
Chen, Q. W. et al. Self-mineralized photothermal bacteria hybridizing with mitochondria-targeted metal–organic frameworks for augmenting photothermal tumor therapy. Adv. Funct. Mater. 30, 1909806 (2020).
Wang, J. W. et al. A self-driven bioreactor based on bacterium–metal–organic framework biohybrids for boosting chemotherapy via lactate catabolism. ACS Nano 15, 17870–17884 (2021).
Wang, X. N., Niu, M. T., Fan, J. X., Chen, Q. W. & Zhang, X. Z. Photoelectric bacteria enhance the in situ production of tetrodotoxin for antitumor therapy. Nano Lett. 21, 4270–4279 (2021).
Li, W. P., Long, X., Kataoka-Hamai, C. & Okamoto, A. Membrane integrated liposome synthesized by a liposome fusion-induced membrane exchange. Preprint at https://doi.org/10.26434/chemrxiv-2022-9tt9m (2022).
Choi, W., Choi, J. Y. & Song, H. Regulation of electron–hole recombination kinetics on uniform metal–semiconductor nanostructures for photocatalytic hydrogen evolution. APL Mater. 7, 100702 (2019).
Chen, L. et al. Composition tunability of semiconductor radiosensitizers for low-dose X-ray induced photodynamic therapy. J. Nanobiotechnol. 20, 293 (2022).
Moon, J. T., Lee, S. K. & Joo, J. B. Controllable one-pot synthesis of uniform colloidal TiO2 particles in a mixed solvent solution for photocatalysis. Beilstein J. Nanotechnol. 9, 1715–1727 (2018).
Zhu, L. J. et al. Ligand-free rutile and anatase TiO2 nanocrystals as electron extraction layers for high performance inverted polymer solar cells. RSC Adv. 7, 20084–20092 (2017).
Mihaly, J. et al. Characterization of extracellular vesicles by IR spectroscopy: fast and simple classification based on amide and C–H stretching vibrations. Biochim. Biophys. Acta Biomembr. 1859, 459–466 (2017).
Bonechi, C. et al. Physicochemical characterization of hyaluronic acid and chitosan liposome. Coat. Appl. Sci. 11, 12071 (2021).
Reuillard, B. et al. High performance reduction of H2O2 with an electron transport decaheme cytochrome on a porous ITO electrode. J. Am. Chem. Soc. 139, 3324–3327 (2017).
Okamoto, A., Nakamura, R. & Hashimoto, K. In-vivo identification of direct electron transfer from Shewanella oneidensis MR-1 to electrodes via outer-membrane OmcA–MtrCAB protein complexes. Electrochim. Acta 56, 5526–5531 (2011).
Okamoto, A., Tokunou, Y., Kalathil, S. & Hashimoto, K. Proton transport in the outer-membrane flavocytochrome complex limits the rate of extracellular electron transport. Angew. Chem. Int. Ed. 56, 9082–9086 (2017).
Van Acker, H. et al. The role of reactive oxygen species in antibiotic-induced cell death in Burkholderia cepacia complex bacteria. PLoS ONE 11, e0159837 (2016).
Shoji, T. et al. DMPO–OH radical formation from 5,5-dimethyl-1-pyrroline N-oxide (DMPO) in hot water. Anal. Sci. 23, 219–221 (2007).
Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 64, 97–112 (1995).
Oberley, L. W. & Buettner, G. R. Role of superoxide-dismutase in cancer—review. Cancer Res. 39, 1141–1149 (1979).
Marklund, S. L., Westman, N. G., Lundgren, E. & Roos, G. Copper-containing and zinc-containing superoxide-dismutase, manganese-containing superoxide-dismutase, catalase, and glutathione-peroxidase in normal and neoplastic human cell-lines and normal human-tissues. Cancer Res. 42, 1955–1961 (1982).
Huang, P., Feng, L., Oldham, E. A., Keating, M. J. & Plunkett, W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature 407, 390–395 (2000).
Clark, J. M. The 3Rs in research: a contemporary approach to replacement, reduction and refinement. Br. J. Nutr. 120, S1–S7 (2018).
Acknowledgements
C.-S.Y. appreciates the financial support provided by National Science and Technology Council, Taiwan (109-2113-M-006 -011-MY3). This research was also supported in part by Higher Education Sprout Project, Ministry of Education to the Headquarters of University Advancement at National Cheng Kung University. Additional financially support was provided by the Center of Applied Nanomedicine, National Cheng Kung University under the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project of the Ministry of Education (MOE) in Taiwan. W.-P.L. appreciates the financial support by the National Science and Technology Council, Taiwan (NSTC 109-2113-M-037-017-MY3 and NSTC 111-2320-B-037-035), Kaohsiung Medical University Research Foundation (KMU-Q111002) and the Yushan Young Scholar Program of the Ministry of Education of Taiwan. C.-H.S. thanks the financial support by the National Science and Technology Council, Taiwan (NSTC 109-2314-B-182-011-MY3; 111-2811-B-182-033-) and the Chang Gung Medical Foundation (CMRPG8M1021-3 and CMRPG8M0121-3). This work was also supported by a Grant-in-Aid for Research from the Japan Society for Promotion of Science KAKENHI (grant number 22H02265); PRIME, the Japan Agency for Medical Research and Development (grant number 19gm6010002h0004); and JST, PRESTO (grant number JPMJPR19H1). We thank the equipment support provided by the Core Research Laboratory, College of Medicine, National Cheng Kung University, and the Bioimaging Core Facility of the National Core Facility for Biopharmaceuticals, National Science and Technology Council, Taiwan. We also thank W.-T. Chiu for his constructive comments on this work.
Author information
Authors and Affiliations
Contributions
C.-S.Y. conceived the research. W.-P.L., C.-H.S., A.O. and C.-S.Y. designed the experiments and supervised the whole project. Y.-C.C., Y.-T.L. and Y.-T.K. synthesized and characterized the TiO2 NPs. W.-P.L., X.L. and A.O. provided the LIME technique and all bacteria. Y.-C.C., Y.-T.L., C.-L.H., M.-C.H, X.L., J.-S.C. and Y.-T.K. fabricated the liposomes and MILs. C.-L.H., M.-C.H. and X.L. contributed to the protein characterization analysis. W.-P.L., A.O. and C.-S.Y. carried out the mechanism analysis. Y.-C.C. and Y.-T.L. performed experiments in the evaluation of ROS generation and in vitro studies. C.-H.S. designed the experiments of animal disease model. C.-L.L., W.-C.L. and C.-H.S. helped with experiments in colony assay and in vivo studies. Y.-C.C., W.-P.L., C.-H.S., A.O. and C.-S.Y. wrote the paper. All the authors have discussed the experimental data, reviewed the paper and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Stefanie Klein and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion and Supplementary Figs. 1–15.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed gels.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, YC., Li, YT., Lee, CL. et al. Electroactive membrane fusion-liposome for increased electron transfer to enhance radiodynamic therapy. Nat. Nanotechnol. 18, 1492–1501 (2023). https://doi.org/10.1038/s41565-023-01476-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01476-2