Abstract
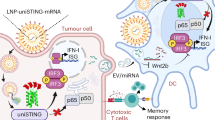
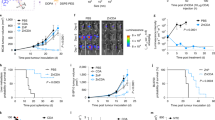
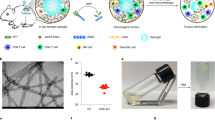
Intravenously administered cyclic dinucleotides and other STING agonists are hampered by low cellular uptake and poor circulatory half-life. Here we report the covalent conjugation of cyclic dinucleotides to poly(β-amino ester) nanoparticles through a cathepsin-sensitive linker. This is shown to increase stability and loading, thereby expanding the therapeutic window in multiple syngeneic tumour models, enabling the study of how the long-term fate of the nanoparticles affects the immune response. In a melanoma mouse model, primary tumour clearance depends on the STING signalling by host cells—rather than cancer cells—and immune memory depends on the spleen. The cancer cells act as a depot for the nanoparticles, releasing them over time to activate nearby immune cells to control tumour growth. Collectively, this work highlights the importance of nanoparticle structure and nano-biointeractions in controlling immunotherapy efficacy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analysed that support the findings of this study are available within this Article and its Supplementary Information. All raw data from this study are available from the corresponding author upon request. Source data are provided with this paper.
References
Yum, S., Li, M., Frankel, A. E. & Chen, Z. J. Roles of the cGAS-STING pathway in cancer immunosurveillance and immunotherapy. Annu. Rev. Cancer Biol. 3, 323–344 (2019).
Kwon, J. & Bakhoum, S. F. The cytosolic DNA-sensing cGAS–STING pathway in cancer. Cancer Discov. 10, 26 (2020).
Ishikawa, H., Ma, Z. & Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009).
Barber, G. N. STING: infection, inflammation and cancer. Nat. Rev. Immunol. 15, 760–770 (2015).
Zitvogel, L., Galluzzi, L., Kepp, O., Smyth, M. J. & Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 15, 405–414 (2015).
Woo, S.-R. et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842 (2014).
Nicolai, C. J. et al. NK cells mediate clearance of CD8+ T cell–resistant tumors in response to STING agonists. Sci. Immunol. 5, eaaz2738 (2020).
Nakamura, T. et al. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J. Immunother. Cancer 9, e002852 (2021).
Fu, J. et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 7, 283ra252 (2015).
Dosta, P. et al. Delivery of stimulator of interferon genes (STING) agonist using polypeptide-modified dendrimer nanoparticles in the treatment of melanoma. Adv. NanoBiomed Res. 1, 2100006 (2021).
Ablasser, A. et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 498, 380–384 (2013).
Wu, J. et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830 (2013).
Zhang, X. et al. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell 51, 226–235 (2013).
Lee, S. E. et al. Improvement of STING-mediated cancer immunotherapy using immune checkpoint inhibitors as a game-changer. Cancer Immunol. Immunother. 71, 3029–3042 (2022).
Jneid, B. et al. Selective STING stimulation in dendritic cells primes antitumor T cell responses. Sci. Immunol. 8, eabn6612 (2023).
Wang, H. et al. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc. Natl Acad. Sci. USA 114, 1637–1642 (2017).
Meric-Bernstam, F. et al. Phase Ib study of MIW815 (ADU-S100) in combination with spartalizumab (PDR001) in patients (pts) with advanced/metastatic solid tumors or lymphomas. J. Clin. Oncol. 37, 2507 (2019).
Harrington, K. J. et al. Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas. Ann. Oncol. 29, VIII712 (2018).
Shae, D. et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 14, 269–278 (2019).
Watkins-Schulz, R. et al. A microparticle platform for STING-targeted immunotherapy enhances natural killer cell- and CD8+ T cell-mediated anti-tumor immunity. Biomaterials 205, 94–105 (2019).
Koshy, S. T., Cheung, A. S., Gu, L., Graveline, A. R. & Mooney, D. J. Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv. Biosyst. 1, 1600013 (2017).
Lin, Z. P. et al. Macrophages actively transport nanoparticles in tumors after extravasation. ACS Nano 16, 6080–6092 (2022).
Miller, M. A. et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat. Commun. 6, 8692 (2015).
Korangath, P. et al. Nanoparticle interactions with immune cells dominate tumor retention and induce T cell–mediated tumor suppression in models of breast cancer. Sci. Adv. 6, eaay1601 (2020).
Dane, E. L. et al. STING agonist delivery by tumour-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. Nat. Mater. 21, 710–720 (2022).
Sun, X. et al. Amplifying STING activation by cyclic dinucleotide–manganese particles for local and systemic cancer metalloimmunotherapy. Nat. Nanotechnol. 16, 1260–1270 (2021).
Riley, R. S., June, C. H., Langer, R. & Mitchell, M. J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 18, 175–196 (2019).
Wehbe, M. et al. Nanoparticle delivery improves the pharmacokinetic properties of cyclic dinucleotide STING agonists to open a therapeutic window for intravenous administration. J. Control. Release 330, 1118–1129 (2021).
Lewis, S. M., Williams, A. & Eisenbarth, S. C. Structure and function of the immune system in the spleen. Sci. Immunol. 4, eaau6085 (2019).
Bronte, V. & Pittet, MikaelJ. The spleen in local and systemic regulation of immunity. Immunity 39, 806–818 (2013).
Segovia, N., Dosta, P., Cascante, A., Ramos, V. & Borrós, S. Oligopeptide-terminated poly(β-amino ester)s for highly efficient gene delivery and intracellular localization. Acta Biomater. 10, 2147–2158 (2014).
Dosta, P., Ramos, V. & Borrós, S. Stable and efficient generation of poly(β-amino ester)s for RNAi delivery. Mol. Syst. Des. Eng. 3, 677–689 (2018).
Dosta, P. et al. Delivery of anti-microRNA-712 to inflamed endothelial cells using poly(beta-amino ester) nanoparticles conjugated with VCAM-1 targeting peptide. Adv. Healthcare Mater. 10, 2001894 (2021).
Nunez-Toldra, R. et al. Improvement of osteogenesis in dental pulp pluripotent-like stem cells by oligopeptide-modified poly(beta-amino ester)s. Acta Biomater. 53, 152–164 (2017).
Dosta, P. et al. Delivery of siRNA to endothelial cells in vivo using lysine/histidine oligopeptide-modified poly(beta-amino ester) nanoparticles. Cardiovasc. Eng. Technol. 12, 114–125 (2021).
Dosta, P., Segovia, N., Cascante, A., Ramos, V. & Borrós, S. Surface charge tunability as a powerful strategy to control electrostatic interaction for high efficiency silencing, using tailored oligopeptide-modified poly(beta-amino ester)s (PBAEs). Acta Biomater. 20, 82–93 (2015).
Puigmal, N., Ramos, V., Artzi, N. & Borrós, S. Poly(β-amino ester)s-based delivery systems for targeted transdermal vaccination. Pharmaceutics 15, 1262 (2023).
Vyskocil, S. et al. Identification of novel carbocyclic pyrimidine cyclic dinucleotide STING agonists for antitumor immunotherapy using systemic intravenous route. J. Med. Chem. 64, 6902–6923 (2021).
Alouane, A., Labruère, R., Le Saux, T., Schmidt, F. & Jullien, L. Self-immolative spacers: kinetic aspects, structure–property relationships, and applications. Angew. Chem. Int. Ed. 54, 7492–7509 (2015).
Bargh, J. D., Isidro-Llobet, A., Parker, J. S. & Spring, D. R. Cleavable linkers in antibody–drug conjugates. Chem. Soc. Rev. 48, 4361–4374 (2019).
Gandini, A. The furan/maleimide Diels–Alder reaction: a versatile click–unclick tool in macromolecular synthesis. Prog. Polym. Sci. 38, 1–29 (2013).
Froidevaux, V. et al. Study of the Diels–Alder and retro-Diels–Alder reaction between furan derivatives and maleimide for the creation of new materials. RSC Adv. 5, 37742–37754 (2015).
Harris, J. M. & Chess, R. B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2, 214–221 (2003).
Fornaguera, C. et al. mRNA delivery system for targeting antigen-presenting cells in vivo. Adv. Healthcare Mater. 7, 1800335 (2018).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Akdis, C. A. & Blaser, K. Mechanisms of interleukin-10-mediated immune suppression. Immunology 103, 131–136 (2001).
Brown, M. A. & Hural, J. Functions of IL-4 and control of its expression. Crit. Rev. Immunol. 17, 1–32 (1997).
Goswami, R. & Kaplan, M. H. A brief history of IL-9. J. Immunol. 186, 3283 (2011).
Harlin, H. et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 69, 3077–3085 (2009).
Sivick, K. E. et al. Magnitude of therapeutic STING activation determines CD8+ T cell-mediated anti-tumor immunity. Cell Rep. 25, 3074–3085.e3075 (2018).
Lechner, M. G. et al. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J. Immunother. 36, 477–489 (2013).
Fitzgerald-Bocarsly, P., Dai, J. & Singh, S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev. 19, 3–19 (2008).
Liang, H. et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat. Commun. 8, 1736 (2017).
Spitzer, M. H. et al. Systemic immunity is required for effective cancer immunotherapy. Cell 168, 487–502.e415 (2017).
Poncette, L., Bluhm, J. & Blankenstein, T. The role of CD4 T cells in rejection of solid tumors. Curr. Opin. Immunol. 74, 18–24 (2022).
Schadt, L. et al. Cancer-cell-intrinsic cGAS expression mediates tumor immunogenicity. Cell Rep. 29, 1236–1248.e1237 (2019).
Carozza, J. A. et al. Extracellular cGAMP is a cancer-cell-produced immunotransmitter involved in radiation-induced anticancer immunity. Nat. Cancer 1, 184–196 (2020).
Madaan, A., Verma, R., Singh, A. T., Jain, S. K. & Jaggi, M. A stepwise procedure for isolation of murine bone marrow and generation of dendritic cells. J. Biol. Methods 1, e1 (2014).
Acknowledgements
We are grateful for the support and funding of Takeda Pharmaceuticals. We thank the Hale Building for Transformative Medicine, the Koch Institute for Integrative Cancer Research at the Massachusetts Institute of Technology (MIT) and at Takeda Boston for the assistance with animal housing. We also thank A. M. Hayward and P. Chamberlain from the Department of Comparative Medicine at MIT for animal assistance, G. Paradis for FACS assistance with Cancer Center Support (FACS core) and K. Cormier for histology assistance.
Author information
Authors and Affiliations
Contributions
P.D., A.O.A.-Y. and N.A. conceived the study. P.D. designed the experiments. S.P.L., D.L., J.W. and S.H. performed the ML-317 synthesis and quantification. P.D., A.M.C., M.Z.D., D.L., J.W., S.H., G.M.T., N.P., S.F., M.P. and A.L.R. performed the in vitro experiments. P.D., A.M.C., S.K., T.H., M.L.G. and V.A.A. performed the in vivo experiments. P.D., A.M.C., M.Z.D. and N.A. drafted and finalized the manuscript with input from all other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Jordan Green, Jason Luke and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–51, discussion and uncropped scans of all blots and gels.
Source data
Source Data Fig. 1
Raw data.
Source Data Fig. 2
Raw data.
Source Data Fig. 3
Raw data.
Source Data Fig. 4
Raw data.
Source Data Fig. 5
Raw data.
Source Data Fig. 6
Raw data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dosta, P., Cryer, A.M., Dion, M.Z. et al. Investigation of the enhanced antitumour potency of STING agonist after conjugation to polymer nanoparticles. Nat. Nanotechnol. 18, 1351–1363 (2023). https://doi.org/10.1038/s41565-023-01447-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01447-7
This article is cited by
-
Targeted delivery of anti-tumour STING agonist
Nature Reviews Bioengineering (2023)