Abstract
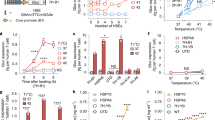
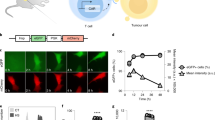
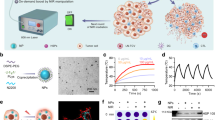
Adoptive T-cell therapy against solid tumours is limited by the apoptosis resistance mechanisms of tumour cells and by the extracellular, immunosuppressive tumour microenvironment. Here we report a temperature-sensitive genome-editing nanodevice that can deliver a Cas9 editor with an external trigger which can be used to edit the genome of tumour cells to reduce resistance to apoptosis and modulate the tumour microenvironment via a mild heating trigger. After local or systemic delivery of Cas9, mild heating is induced by non-invasive near-infrared (NIR) light or focused ultrasound (FUS) to activate Cas9, which initiates simultaneous genome editing of HSP70 (HSPA1A) and BAG3 in tumour cells. This disrupts the apoptotic resistance machinery of the tumour cells against adoptive T cells. At the same time, an NIR- or FUS-induced mild thermal effect reshapes the extracellular tumour microenvironment by disrupting the physical barriers and immune suppression. This facilitates the infiltration of adoptive T cells and enhances their therapeutic activity. Mild thermal Cas9 delivery is demonstrated in different murine tumour models which mimic a range of clinical indications, including a tumour model based on humanized patient-derived xenografts. As a result, the non-invasive thermal delivery of Cas9 significantly enhances the therapeutic efficacies of tumour-infiltrating lymphocytes and chimeric antigen receptor T and shows potential for clinical application.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source data are available for Figs. 1–6 and Supplementary Figures 2–28 in the associated source data files. The raw data of tumour transcriptome sequencing were deposited at the National Center for Biotechnology Information (NCBI) under BioProject: PRJNA916759. The raw data of deep sequencing were deposited at the NCBI under BioProject: PRJNA930575. Source data are provided with this paper.
References
Hou, A. J., Chen, L. C. & Chen, Y. Y. Navigating CAR-T cells through the solid-tumor microenvironment. Nat. Rev. Drug Discov. 20, 531–550 (2021).
Hong, M., Clubb, J. D. & Chen, Y. Y. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell 38, 473–488 (2020).
Chen, J. et al. NR4A transcription factors limit CAR T cell function in solid tumors. Nature 567, 530–534 (2019).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Zou, W. Immunosuppressive networks in the tumor environment and their therapeutic relevance. Nat. Rev. Cancer 5, 263–274 (2005).
Huang, Y. et al. Improving immune–vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 18, 195–203 (2018).
Caruana, I. et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 21, 524–529 (2015).
Chang, Z. L., Hou, A. J. & Chen, Y. Y. Engineering primary T cells with chimeric antigen receptors for rewired responses to soluble ligands. Nat. Protoc. 15, 1507–1524 (2020).
Leen, A. M. et al. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol. Ther. 22, 1211–1220 (2014).
Cherkassky, L. et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 126, 3130–3144 (2016).
Liu, X. et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T-cells in advanced solid tumors. Cancer Res. 76, 1578–1590 (2016).
Tang, T. C. Y., Xu, N. & Dolnikov, A. Targeting the immune-suppressive tumor microenvironment to potentiate CAR T cell therapy. Cancer Rep. Rev. 4, 1–5 (2020).
Karlsson, H. Approaches to augment CAR T-cell therapy by targeting the apoptotic machinery. Biochem. Soc. Trans. 44, 371–376 (2016).
Green, D. R. The coming decade of cell death research: five riddles. Cell 177, 1094–1107 (2019).
Jorgensen, I., Rayamajhi, M. & Miao, E. A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 17, 151–164 (2017).
Kim, J. A., Kim, Y., Kwon, B. M. & Han, D. C. The natural compound cantharidin induces cancer cell death through inhibition of heat shock protein 70 (HSP70) and BCL2-associated athanogene domain 3 (BAG3) expression by blocking heat shock factor 1 (HSF1) binding to promoters. J. Biol. Chem. 288, 28713–28726 (2013).
Rosati, A., Graziano, V., Laurenzi, V. D., Pascale, M. & Turco, M. C. BAG3: a multifaceted protein that regulates major cell pathways. Cell Death Dis. 2, e141 (2011).
Wang, B. K. et al. Gold-nanorods–siRNA nanoplex for improved photothermal therapy by gene silencing. Biomaterials 78, 27 (2016).
Joung, J. et al. CRISPR activation screen identifies BCL-2 proteins and B3GNT2 as drivers of cancer resistance to T cell-mediated cytotoxicity. Nat. Commun. 13, 1606 (2022).
Rosati, A. et al. BAG3 promotes pancreatic ductal adenocarcinoma growth by activating stromal macrophages. Nat. Commun. 6, 8695 (2015).
Lamprecht, A. Nanomedicines in gastroenterology and hepatology. Nat. Rev. Gastroenterol. Hepatol. 12, 669 (2015).
Dudeja, V., Vickers, S. M. & Saluja, A. K. The role of heat shock proteins in gastrointestinal diseases. Gut 58, 1000–1009 (2009).
Marzullo, L., Turco, M. C. & Marco, M. D. The multiple activities of BAG3 protein: mechanisms. Biochim. Biophys. Acta, Gen. Subj. 1864, 129628 (2020).
Romano, M. F. et al. BAG3 protein controls B-chronic lymphocytic leukaemia cell apoptosis. Cell Death Differ. 10, 383–385 (2003).
Ammirante, M. et al. IKKγ protein is a target of BAG3 regulatory activity in human tumor growth. Proc. Natl Acad. Sci. USA 107, 7497–7502 (2010).
Eltoukhy, A. A., Chen, D., Albi, C. A., Langer, R. & Anderson, D. G. Degradable terpolymers with alkyl side chains demonstrate enhanced gene delivery potency and nanoparticle stability. Adv. Mater. 25, 1487–1493 (2013).
Rui, Y. et al. High-throughput and high-content bioassay enables tuning of polyester nanoparticles for cellular uptake, endosomal escape, and systemic in vivo delivery of mRNA. Sci. Adv. 8, eabk2855 (2022).
Zha, M. et al. An ester-substituted semiconducting polymer with efficient nonradiative decay enhances NIR-II photoacoustic performance for monitoring of tumor growth. Angew. Chem. Int. Ed. 59, 23268–23276 (2020).
Banerjee, R., Tyagi, P., Li, S. & Huang, L. Anisamide-targeted stealth liposomes: a potent carrier for targeting doxorubicin to human prostate cancer cells. Int. J. Cancer 112, 693–700 (2004).
Chen, Y. et al. Delivery of CRISPR/Cas9 plasmids by cationic gold nanorods: impact of the aspect ratio on genome editing and treatment of hepatic fibrosis. Chem. Mater. 33, 81–91 (2021).
Li, N. et al. Chimeric antigen receptor-modified T cells redirected to EphA2 for the immunotherapy of non-small cell lung cancer. Transl. Oncol. 11, 11–17 (2018).
Chen, X., Chen, Y., Xin, H., Wan, T. & Ping, Y. Near-infrared optogenetic engineering of photothermal nanoCRISPR for programmable genome editing. Proc. Natl Acad. Sci. USA 117, 2395–2405 (2020).
Chen, Y., Yan, X. & Ping, Y. Optical manipulation of CRISPR/Cas9 functions: from ultraviolet to near-infrared light. ACS Mater. Lett. 2, 644–653 (2020).
Zhang, W., He, M., Huang, G. & He, J. A comparison of ultrasound-guided high intensity focused ultrasound for the treatment of uterine fibroids in patients with an anteverted uterus and a retroverted uterus. Int. J. Hyperther. 32, 623–629 (2016).
Klichinsky, M. et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 38, 947–953 (2020).
Guo, Y. et al. Metabolic reprogramming of terminally exhausted CD8+ T cells by IL-10 enhances anti-tumor immunity. Nat. Immunol. 22, 746–756 (2021).
Etxeberria, I. et al. Intratumor adoptive transfer of IL-12 mRNA transiently engineered antitumor CD8+ T cells. Cancer Cell 36, 613–629 (2019).
Singh, N. et al. Antigen-independent activation enhances the efficacy of 4-1BB-costimulated CD22 CAR T cells. Nat. Med. 27, 842–850 (2021).
Etxeberria, I. et al. Engineering bionic T cells: signal 1, signal 2, signal 3, reprogramming and the removal of inhibitory mechanisms. Cell. Mol. Immunol. 17, 576–586 (2020).
Rostamian, H. et al. A metabolic switch to memory CAR T cells: implications for cancer treatment. Cancer Lett. 500, 107–118 (2021).
Korde, L. A., Somerfield, M. R. & Hershman, D. L. Use of immune checkpoint inhibitor pembrolizumab in the treatment of high-risk, early-stage triple-negative breast cancer: ASCO guideline rapid recommendation update. J. Clin. Oncol. 39, 1696–1698 (2021).
Yoshida, K., Yamaguchi, K., Okumura, N., Tanahashi, T. & Kodera, Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer 19, 329–338 (2016).
Song, T., Lang, M., Ren, S., Gan, L. & Lu, W. The past, present and future of conversion therapy for liver cancer. Am. J. Cancer Res. 11, 4711–4724 (2021).
Sun, H. & Zhu, X. Downstaging conversion therapy in patients with initially unresectable advanced hepatocellular carcinoma: an overview. Front. Oncol. 11, 772195 (2021).
Kishton, R. J., Lynn, R. C. & Restifo, N. P. Strength in numbers: identifying neoantigen targets for cancer immunotherapy. Cell 184, 5031–5052 (2021).
Storz, P. & Crawford, H. C. Carcinogenesis of pancreatic ductal adenocarcinoma. Gastroenterology 158, 2072–2081 (2020).
Hosein, A. N., Dougan, S. K., Aguirre, A. J. & Maitra, A. Translational advances in pancreatic ductal adenocarcinoma therapy. Nat. Cancer 3, 272–286 (2022).
Xue, G. et al. Adoptive cell therapy with tumor-specific Th9 cells induces viral mimicry to eliminate antigen-loss-variant tumor cells. Cancer Cell 39, 1610–1622 (2021).
Hirabayashi, K. et al. Dual targeting CAR-T cells with optimal costimulation and metabolic fitness enhance antitumor activity and prevent escape in solid tumors. Nat. Cancer 2, 904–918 (2021).
Bergers, G. & Fendt, S. The metabolism of cancer cells during metastasis. Nat. Rev. Cancer 21, 162–180 (2021).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFA0909900, Y.P.), the National Natural Science Foundation of China (82073779, 32261143727, Y.P.; U2001224, W.W.; U21A2097, K.L.) and the Natural Science Foundation of Zhejiang Province (Distinguished Young Scholar Program, LR21H300002, Y.P.). We thank H.-Y. Lai and T. He for their kind assistance with the FUS experiments.
Author information
Authors and Affiliations
Contributions
X.C., S.W. and Y.C. designed and performed experiments and analysed and interpreted data. H.X. and S.Z. constructed the plasmid and analysed deep-sequencing results. Y.X. helped construct the PDX model. M.Z. and K.L. synthesized the BDT-TQE semiconducting polymer. D.W., H.L. and Z.G. discussed the manuscript. W.W. and Y.P. conceived the idea, designed the study, analysed and interpreted data, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Z.G. is a scientific cofounder of ZenCapsule and ZCapsule. Y.P. is a scientific cofounder of Ruidax. The other authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Kanyi Pu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, figs. 1–28, tables 1–3, references, and unprocessed blots and gels for supplementary figures.
Supplementary Data 1
Supplementary statistical source data.
Source data
Source Data Fig. 1
Unprocessed western blots and gels, and statistical data for Fig. 1.
Source Data Fig. 2
Unprocessed western blots and gels, and statistical data for Fig. 2.
Source Data Fig. 3
Statistical data for Fig. 3.
Source Data Fig. 4
Unprocessed western blots and gels, and statistical data for Fig. 4.
Source Data Fig. 5
Unprocessed western blots and gels, and statistical data for Fig. 5.
Source Data Fig. 6
Unprocessed western blots and gels, and statistical data for Fig. 6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Wang, S., Chen, Y. et al. Non-invasive activation of intratumoural gene editing for improved adoptive T-cell therapy in solid tumours. Nat. Nanotechnol. 18, 933–944 (2023). https://doi.org/10.1038/s41565-023-01378-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01378-3