Abstract
Ion-selective channels play a key role in physiological processes and are used in many technologies. Although biological channels can efficiently separate same-charge ions with similar hydration shells, it remains a challenge to mimic such exquisite selectivity using artificial solid-state channels. Although there are several nanoporous membranes that show high selectivity with respect to certain ions, the underlying mechanisms are based on the hydrated ion size and/or charge. There is a need to rationalize the design of artificial channels to make them capable of selecting between similar-sized same-charge ions, which, in turn, requires an understanding of why and how such selectivity can occur. Here we study ångström-scale artificial channels made by van der Waals assembly, which are comparable in size with typical ions and carry little residual charge on the channel walls. This allows us to exclude the first-order effects of steric- and Coulomb-based exclusion. We show that the studied two-dimensional ångström-scale capillaries can distinguish between same-charge ions with similar hydrated diameters. The selectivity is attributed to different positions occupied by ions within the layered structure of nanoconfined water, which depend on the ion-core size and differ for anions and cations. The revealed mechanism points at the possibilities of ion separation beyond simple steric sieving.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article, extended data and its Supplementary Information. Source data are provided with this paper.
References
Nightingale, E. R. Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 63, 1381–1387 (1959).
Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 86, 119–126 (2012).
Speight, J. Lange’s Handbook of Chemistry 16th edn (McGraw Hill Education, 2005).
Jentsch, T. J. & Günther, W. Chloride channels: an emerging molecular picture. BioEssays 19, 117–126 (1997).
Hille, B. Ion Channels of Excitable Membranes (Sinauer, 2001).
Dudev, T. & Lim, C. Factors governing the Na+ vs K+ selectivity in sodium ion channels. J. Am. Chem. Soc. 132, 2321–2332 (2010).
Lj, M. The penetration of some cations into muscle. J. Gen. Physiol. 42, 817–829 (1959).
Doyle, D. A. et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77 (1998).
Sahu, S. & Zwolak, M. Colloquium: ionic phenomena in nanoscale pores through 2D materials. Rev. Mod. Phys. 91, 021004 (2019).
Epsztein, R., DuChanois, R. M., Ritt, C. L., Noy, A. & Elimelech, M. Towards single-species selectivity of membranes with subnanometre pores. Nat. Nanotechnol. 15, 426–436 (2020).
Gao, J., Feng, Y., Guo, W. & Jiang, L. Nanofluidics in two-dimensional layered materials: inspirations from nature. Chem. Soc. Rev. 46, 5400–5424 (2017).
Wang, L. et al. Fundamental transport mechanisms, fabrication and potential applications of nanoporous atomically thin membranes. Nat. Nanotechnol. 12, 509–522 (2017).
Epsztein, R., Shaulsky, E., Dizge, N., Warsinger, D. M. & Elimelech, M. Role of ionic charge density in Donnan exclusion of monovalent anions by nanofiltration. Environ. Sci. Technol. 52, 4108–4116 (2018).
Faucher, S. et al. Critical knowledge gaps in mass transport through single-digit nanopores: a review and perspective. J. Phys. Chem. C 123, 21309–21326 (2019).
Thiruraman, J. P., Masih Das, P. & Drndić, M. Ions and water dancing through atom-scale holes: a perspective toward ‘size zero’. ACS Nano 14, 3736–3746 (2020).
Karahan, H. E. et al. MXene materials for designing advanced separation membranes. Adv. Mater. 32, 1906697 (2020).
Feng, J. et al. Observation of ionic Coulomb blockade in nanopores. Nat. Mater. 15, 850–855 (2016).
Ma, J. et al. Drastically reduced ion mobility in a nanopore due to enhanced pairing and collisions between dehydrated ions. J. Am. Chem. Soc. 141, 4264–4272 (2019).
Fu, Y. et al. Dehydration-determined ion selectivity of graphene subnanopores. ACS Appl. Mater. Interfaces 12, 24281–24288 (2020).
Li, X. et al. Fast and selective fluoride ion conduction in sub-1-nanometer metal–organic framework channels. Nat. Commun. 10, 2490 (2019).
Xin, W. et al. Biomimetic KcsA channels with ultra-selective K+ transport for monovalent ion sieving. Nat. Commun. 13, 1701 (2022).
You, Y. et al. Angstrofluidics: walking to the limit. Annu. Rev. Mater. Res. 52, 189–218 (2022).
Esfandiar, A. et al. Size effect in ion transport through angstrom-scale slits. Science 358, 511–513 (2017).
Gopinadhan, K. et al. Complete steric exclusion of ions and proton transport through confined monolayer water. Science 363, 145–148 (2019).
Radha, B. et al. Molecular transport through capillaries made with atomic-scale precision. Nature 538, 222–225 (2016).
Haynes, W. M. CRC Handbook of Chemistry and Physics 96th edn (CRC Press, 2003).
Rollings, R. C., Kuan, A. T. & Golovchenko, J. A. Ion selectivity of graphene nanopores. Nat. Commun. 7, 11408 (2016).
Hong, S. et al. Scalable graphene-based membranes for ionic sieving with ultrahigh charge selectivity. Nano Lett. 17, 728–732 (2017).
Perram, J. W. & Stiles, P. J. On the nature of liquid junction and membrane potentials. Phys. Chem. Chem. Phys. 8, 4200–4213 (2006).
Yu, Y. et al. Charge asymmetry effect in ion transport through angstrom-scale channels. J. Phys. Chem. C 123, 1462–1469 (2019).
Razmjou, A. et al. Lithium ion-selective membrane with 2D subnanometer channels. Water Res. 159, 313–323 (2019).
Bajaj, P., Richardson, J. O. & Paesani, F. Ion-mediated hydrogen-bond rearrangement through tunnelling in the iodide–dihydrate complex. Nat. Chem. 11, 367–374 (2019).
Tocci, G., Joly, L. & Michaelides, A. Friction of water on graphene and hexagonal boron nitride from ab initio methods: very different slippage despite very similar interface structures. Nano Lett. 14, 6872–6877 (2014).
Grosjean, B. et al. Chemisorption of hydroxide on 2D materials from DFT calculations: graphene versus hexagonal boron nitride. J. Phys. Chem. Lett. 7, 4695–4700 (2016).
Robin, P., Kavokine, N. & Bocquet, L. Modeling of emergent memory and voltage spiking in ionic transport through angstrom-scale slits. Science 373, 687–691 (2021).
Sajja, R. et al. Hydrocarbon contamination in angström-scale channels. Nanoscale 13, 9553–9560 (2021).
Berendsen, H. J. C., Grigera, J. R. & Straatsma, T. P. The missing term in effective pair potentials. J. Phys. Chem. 91, 6269–6271 (1987).
Koneshan, S., Rasaiah, J. C., Lynden-Bell, R. M. & Lee, S. H. Solvent structure, dynamics, and ion mobility in aqueous solutions at 25 °C. J. Phys. Chem. B 102, 4193–4204 (1998).
Agieienko, V. N., Kolesnik, Y. V. & Kalugin, O. N. Structure, solvation, and dynamics of Mg2+, Ca2+, Sr2+, and Ba2+ complexes with 3-hydroxyflavone and perchlorate anion in acetonitrile medium: a molecular dynamics simulation study. J. Chem. Phys. 140, 194501 (2014).
Lorentz, H. A. Ueber die Anwendung des Satzes vom Virial in der kinetischen Theorie der Gase. Ann. Phys. 248, 127–136 (1881).
Berthelot, D. Sur le mélange des gaz. Compt. Rendus 126, 1703–1706 (1898).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995).
Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 81, 511–519 (1984).
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A 31, 1695–1697 (1985).
Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 113, 7756–7764 (2000).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Delley, B. An all‐electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 92, 508–517 (1990).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Acknowledgements
B.R. acknowledges funding from the Royal Society University Research Fellowship URF\R1\180127 and Philip Leverhulme Prize PLP-2021-262. B.R., S.G., A.I. and Y.Y. acknowledge funding from the European Union’s H2020 Framework Programme/ERC Starting Grant 852674—AngstroCAP, RS enhancement award RF\ERE\210016 and EPSRC strategic equipment grant EP/W006502/1. A.K. acknowledges the Ramsay Memorial Fellowship, Royal Society International Exchanges grant IES\R1\201028 and EPSRC strategic equipment grant EP/W006502/1. F.W. acknowledges the Youth Innovation Promotion Association CAS (2020449) and Hefei Advanced Computing Center. H.J and M.N.-A. acknowledge the high-performance computing support from Shahid Rajaee Teacher Training University.
Author information
Authors and Affiliations
Contributions
B.R. designed and directed the project. A.K., Y.Y. and A.B. carried out the sample fabrication of several Å-channel devices. S.G. and A.I. performed the ion conductance measurements and their analysis. S.G. conducted the diffusion measurements and their analysis. A.K. and M.V.S.M. carried out the sample characterization. H.J., N.H., M.N.-A., Y.L., H.P. and F.W. provided the theoretical simulations. B.R., S.G., A.I. and A.K. wrote the manuscript with inputs from M.N.-A. and F.W. All the authors contributed to discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Nikita Kavokine and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Fabrication process flow.
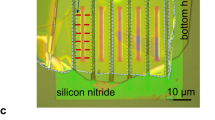
The sequence of fabrication steps is indicated by the arrows. (a) Freestanding silicon nitride (SiNx) membrane (100 × 100 µm2) was prepared by wet etching. (b) Rectangular aperture (typically, 3 × 26 µm2) was made in the membrane using photolithography and dry etching. (c) Bottom 2D crystal was placed on top of the aperture. (d) Strips of bilayer graphene were transferred onto the bottom crystal. Then the two-layer assembly was dry etched from the back with the SiNx aperture serving as a protective mask. (e) Another 2D crystal (top layer) was placed on top to cover the entire etched microhole. (f) A gold stripe was deposited on top of the trilayer assembly. (g) This stripe was used as a mask for subsequent dry etching. The inset shows schematically a cross-section of the resulting Å-channels.
Extended Data Fig. 2 Optical micrographs of Å-channel devices.
(a) Top view of the trilayer assembly (hBN-graphene spacers-hBN) before depositing a gold mask. The graphene spacer is not clearly visible as it is very thin (height, ~0.7 nm). The rectangular microhole in the SiNx membrane is seen in dark blue. Top view of the same device (b) after the gold mask was deposited and (c) after etching away the exposed areas of the 2D crystals.
Extended Data Fig. 3 Equilibrium geometries and electrostatic potential (ESP) map of the hydrated anions.
The optimized structures and the natural bond orbitals (NBO) charges (black numbers) and ion distance to the water molecule (green numbers) of hydrated anions (a) F−, (b) Cl−, (c) I−, and (d) ClO4−in bulk with six water molecules (right hand side, RHS-bottom of each panel) and the hydrated anions between graphene layers (left hand side, LHS-bottom is zoomed in structure of each panel). In each panel, RHS-up is the electrostatic potential energy maps of the hydrated anions. Electrostatic potentials are mapped on the surface of the electron density of 0.002 units.
Extended Data Fig. 4 Equilibrium geometries and electrostatic potential (ESP) map of the hydrated cations.
The optimized structures and the NBO charges (black numbers) and ion distance to the water molecule (green numbers) of hydrated cations (a) Li+, (b) Na+, (c) K+, and (d) Cs+ in bulk with six water molecules (RHS-bottom of each panel) and the hydrated cations between graphene layers (LHS-bottom is zoomed in the structure of each panel). In each panel, RHS-up is the electrostatic potential energy maps of the hydrated cations. Electrostatic potentials are mapped on the surface of the electron density of 0.002 units.
Supplementary information
Supplementary Information
Supplementary Sections 1–9, Figs. 1–11, Tables 1–5 and Discussion.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goutham, S., Keerthi, A., Ismail, A. et al. Beyond steric selectivity of ions using ångström-scale capillaries. Nat. Nanotechnol. 18, 596–601 (2023). https://doi.org/10.1038/s41565-023-01337-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01337-y