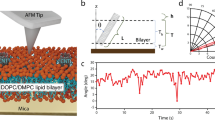
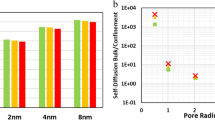
Abstract
For over 100 years, the Nernst–Einstein relation has linked a charged particle’s electrophoretic mobility and diffusion coefficient. Here we report experimental measurements of diffusion and electromigration of K+ ions in narrow 0.8-nm-diameter single-walled carbon nanotube porins (CNTPs) and demonstrate that the Nernst–Einstein relation in these channels breaks down by more than three orders of magnitude. Molecular dynamics simulations using polarizable force fields show that K+ ion diffusion in CNTPs in the presence of a single-file water chain is three orders of magnitude slower than bulk diffusion. Intriguingly, the simulations also reveal a disintegration of the water chain upon application of electric fields, resulting in the formation of distinct K+–water clusters, which then traverse the CNTP at high velocity. Finally, we show that although individual ion–water clusters still obey the Nernst–Einstein relation, the overall relation breaks down because of two distinct mechanisms for ion diffusion and electromigration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data (experimental and theory/computational) reported in this paper have been deposited in FigShare (https://figshare.com) and are available at https://doi.org/10.6084/m9.figshare.20728327.
References
Nernst, W. Zur kinetik der in lösung befindlichen körper (On the kinetics of bodies in solution). Zeit Phys. Chem. 2, 613–637 (1888).
Einstein, A. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen (On the motion of small particels suspended in liquids at rest required by the molecular-kinetic theory of heat). Ann. Phys. 4, 549–560 (1905).
Plawsky, J. L. in Transport Phenomena Fundamentals 4th edn (eds Heinemann, H. & Speight, J. G.) Ch. 3 (CRC Press, 2020).
Weiss, T. F. Cellular Biophysics: Volume 1: Transport (MIT Press, 1996).
Kirby, B. J. in Micro- and Nanoscale Fluid Mechanics: Transport in Microfluidic Devices Ch. 11 (Cambridge Univ. Press, 2010).
Faucher, S. et al. Critical knowledge gaps in mass transport through single-digit nanopores: a review and perspective. J. Phys. Chem. C 123, 21309–21326 (2019).
Daiguji, H., Yang, P. & Majumdar, A. Ion transport in nanofluidic channels. Nano Lett. 4, 137–142 (2004).
Lee, C. et al. Large apparent electric size of solid-state nanopores due to spatially extended surface conduction. Nano Lett. 12, 4037–4044 (2012).
Lin, C.-Y., Combs, C., Su, Y.-S., Yeh, L.-H. & Siwy, Z. S. Rectification of concentration polarization in mesopores leads to high conductance ionic diodes and high performance osmotic power. J. Am. Chem. Soc. 141, 3691–3698 (2019).
Dechadilok, P. & Deen, W. M. Hindrance factors for diffusion and convection in pores. Ind. Eng. Chem. Res. 45, 6953–6959 (2006).
Renkin, E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J. Gen. Physiol. 38, 225–243 (1954).
Cheng, C. et al. Ion transport in complex layered graphene-based membranes with tuneable interlayer spacing. Sci. Adv. 2, e1501272 (2016).
Bason, S., Kaufman, Y. & Freger, V. Analysis of ion transport in nanofiltration using phenomenological coefficients and structural characteristics. J. Phys. Chem. B 114, 3510–3517 (2010).
Wu, J., Gerstandt, K., Zhang, H., Liu, J. & Hinds, B. J. Electrophoretically induced aqueous flow through single-walled carbon nanotube membranes. Nat. Nanotechnol. 7, 133–139 (2012).
Choi, W. et al. Diameter-dependent ion transport through the interior of isolated single-walled carbon nanotubes. Nat. Commun. 4, 2397 (2013).
Fornasiero, F. et al. Ion exclusion by sub-2-nm carbon nanotube pores. Proc. Natl Acad. Sci. USA 105, 17250–17255 (2008).
Esfandiar, A. et al. Size effect in ion transport through angstrom-scale slits. Science 358, 511–513 (2017).
Pang, P., He, J., Park, J. H., Krstić, P. S. & Lindsay, S. Origin of giant ionic currents in carbon nanotube channels. ACS Nano 5, 7277–7283 (2011).
Tunuguntla, R. H. et al. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 357, 792–796 (2017).
Venema, K., Gibrat, R., Grouzis, J.-P. & Grignon, C. Quantitative measurement of cationic fluxes, selectivity and membrane potential using liposomes multilabelled with fluorescent probes. Biochim. Biophys. Acta Biomembr. 1146, 87–96 (1993).
Lokesh, M., Youn, S. K. & Park, H. G. Osmotic transport across surface functionalized carbon nanotube membrane. Nano Lett. 18, 6679–6685 (2018).
Yao, Y.-C. et al. Strong electroosmotic coupling dominates ion conductance of 1.5 nm diameter carbon nanotube porins. ACS Nano 13, 12851–12859 (2019).
Li, Z. et al. Strong differential monovalent anion selectivity in narrow diameter carbon nanotube porins. ACS Nano 14, 6269–6275 (2020).
Haynes, W. M., Lide, D. R. & Bruno, T. J. (eds) CRC Handbook of Chemistry and Physics 97th edn, 75–76 (CRC Press, 2016).
Nightingale, E. Jr Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 63, 1381–1387 (1959).
Misra, R. P. & Blankschtein, D. Insights on the role of many-body polarization effects in the wetting of graphitic surfaces by water. J. Phys. Chem. C 121, 28166–28179 (2017).
Misra, R. P. & Blankschtein, D. Uncovering a universal molecular mechanism of salt ion adsorption at solid/water interfaces. Langmuir 37, 722–733 (2021).
Lamoureux, G. & Roux, B. Modeling induced polarization with classical Drude oscillators: theory and molecular dynamics simulation algorithm. J. Chem. Phys. 119, 3025–3039 (2003).
Hummer, G., Rasaiah, J. C. & Noworyta, J. P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 414, 188–190 (2001).
Corry, B. Designing carbon nanotube membranes for efficient water desalination. J. Phys. Chem. B 112, 1427–1434 (2008).
Corry, B. Water and ion transport through functionalised carbon nanotubes: implications for desalination technology. Energy Environ. Sci. 4, 751–759 (2011).
Mondal, S. & Bagchi, B. Water in carbon nanotubes: pronounced anisotropy in dielectric dispersion and its microscopic origin. J. Phys. Chem. Lett. 10, 6287–6292 (2019).
Loche, P., Ayaz, C., Schlaich, A., Uematsu, Y. & Netz, R. R. Giant axial dielectric response in water-filled nanotubes and effective electrostatic ion–ion interactions from a tensorial dielectric model. J. Phys. Chem. B 123, 10850–10857 (2019).
Secchi, E., Niguès, A., Jubin, L., Siria, A. & Bocquet, L. Scaling behavior for ionic transport and its fluctuations in individual carbon nanotubes. Phys. Rev. Lett. 116, 154501 (2016).
Biesheuvel, P. & Bazant, M. Analysis of ionic conductance of carbon nanotubes. Phys. Rev. E 94, 050601 (2016).
Chipot, C. & Comer, J. Subdiffusion in membrane permeation of small molecules. Sci. Rep. 6, 35913 (2016).
Metzler, R. & Klafter, J. The random walk’s guide to anomalous diffusion: a fractional dynamics approach. Phys. Rep. 339, 1–77 (2000).
Taylor, G. I. Disintegration of water drops in an electric field. Proc. R. Soc. Ser. A 280, 383–397 (1964).
Gao, X., Zhao, T. & Li, Z. Fluid breakup in carbon nanotubes: an explanation of ultrafast ion transport. Phys. Fluids 29, 092003 (2017).
Chen, X. & Gross, R. W. Potassium flux through gramicidin ion channels is augmented in vesicles comprising plasmenylcholine: correlations between gramicidin conformation and function in chemically distinct host bilayer matrixes. Biochemistry 34, 7356–7364 (1995).
Andersen, O. S. Ion movement through gramicidin A channels. Single-channel measurements at very high potentials. Biophys. J. 41, 119–133 (1983).
Hemmler, R., Böse, G., Wagner, R. & Peters, R. Nanopore unitary permeability measured by electrochemical and optical single transporter recording. Biophys. J. 88, 4000–4007 (2005).
Menestrina, G. Ionic channels formed by Staphylococcus aureus alpha-toxin: voltage-dependent inhibition by divalent and trivalent cations. J. Membr. Biol. 90, 177–190 (1986).
Tunuguntla, R. H., Escalada, A., Frolov, V. A. & Noy, A. Synthesis, lipid membrane incorporation, and ion permeability testing of carbon nanotube porins. Nat. Protoc. 11, 2029–2047 (2016).
Tunuguntla, R. H., Allen, F. I., Kim, K., Belliveau, A. & Noy, A. Ultrafast proton transport in sub-1-nm diameter carbon nanotube porins. Nat. Nanotechnol. 11, 639–644 (2016).
Jezek, P., Mahdi, F. & Garlid, K. Reconstitution of the beef heart and rat liver mitochondrial K+/H+ (Na+/H+) antiporter. Quantitation of K+ transport with the novel fluorescent probe, PBFI. J. Biol. Chem. 265, 10522–10526 (1990).
Wanunu, M. et al. Rapid electronic detection of probe-specific microRNAs using thin nanopore sensors. Nat. Nanotechnol. 5, 807–814 (2010).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug‐like molecules compatible with the CHARMM all‐atom additive biological force fields. J. Comp. Chem. 31, 671–690 (2010).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comp. Phys. 117, 1–19 (1995).
Dequidt, A., Devemy, J. & Padua, A. A. Thermalized Drude oscillators with the LAMMPS molecular dynamics simulator. J. Chem. Inf. Model 56, 260–268 (2016).
Kučerka, N. et al. Lipid bilayer structure determined by the simultaneous analysis of neutron and X-ray scattering data. Biophys. J. 95, 2356–2367 (2008).
Grossfield, A. WHAM: The Weighted Histogram Analysis Method Version 2.0.1 (University of Rochester,2021); http://membrane.urmc.rochester.edu/wordpress/?page_id=126
Bonthuis, D. J. et al. Theory and simulations of water flow through carbon nanotubes: prospects and pitfalls. J. Phys. Cond. Matter 23, 184110 (2011).
Vaitheeswaran, S., Rasaiah, J. C. & Hummer, G. Electric field and temperature effects on water in the narrow nonpolar pores of carbon nanotubes. J. Chem. Phys. 121, 7955–7965 (2004).
Shafiei, M., von Domaros, M., Bratko, D. & Luzar, A. Anisotropic structure and dynamics of water under static electric fields. J. Chem. Phys. 150, 074505 (2019).
Acknowledgements
This work was supported as part of the Center for Enhanced Nanofluidic Transport (CENT), an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences under award number DE-SC0019112. Work at the Lawrence Livermore National Laboratory was performed under the auspices of the US Department of Energy under Contract DE-AC52-07NA27344. Z.L. was supported by the Scientific Research Foundation of Graduate School of Southeast University (grant number YBJJ1802), Postgraduate Research and Practice Innovation Program of Jiangsu Province (grant number KYCX18_0067) and China Scholarship Council (CSC 201806090020). R.P.M. and D.B. acknowledge the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by NSF grant number ACI1548562, for computational resources used to develop polarizable force fields, and the Lawrence Livermore National Laboratory for computational resources used to carry out MD simulations of ion transport. The authors also thank Dr. Tuan Anh Pham for facilitating access to LLNL computing resources, and Professor Martin Z. Bazant, Professor Michael S. Strano, and Dr. Satish K. Iyemperumal for fruitful discussions.
Author information
Authors and Affiliations
Contributions
Z.L. and A.N. designed the experiments and R.P.M. and D.B. designed the modelling component involving molecular simulations. Z.L. and Y.L. performed ion diffusion measurements, and S.Z. and Y.-C.Y. performed electrophoretic transport measurements. R.P.M. formulated the theoretical framework involving all-atomistic polarizable force fields and carried out the MD simulations and theoretical analysis. Z.L., R.P.M., Y.L., S.Z., Y.Z., Y.C., D.B. and A.N. analysed the data. A.N. and D.B. directed the project. All authors contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Chih-Jen Shih and Nikita Kavokine for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Potassium ions transport into LUVs in the absence of CNTPs.
A representative stopped-flow kinetic trace recorded for LUVs after exposure to a 25 mM K+ ion concentration gradient. No K+ ion transport was observed on the timescale of the experiment.
Extended Data Fig. 2 Calibration of the fluorescence of the PBFI dye.
a. Changes in the dye fluorescence at a given K+ ion concentration. b. Calibration curve of the PBFI dye in accordance to the calibration equation. The derived value of Fmax from the linear fitting is 2.399 and the dissociation constant Kd is 11.41 mM. Data are shown as mean ± s.d. (n = 3 measurements).
Extended Data Fig. 3 Simultaneous determinations of K+ ion concentrations inside the liposomes.
a. K+ ion concentration kinetics after exposure of CNTP–LUVs to different K+ ion concentration gradients. b. K+ ion concentration kinetics after exposure of control LUVs to a K+ ion concentration gradient of 25 mM.
Extended Data Fig. 4 Ionic conductance of individual carbon nanotube porins.
Example trace showing jumps that correspond to individual CNTPs incorporating and leaving the lipid bilayer formed by the painting method. Data was taken in a buffer containing 100 mM KCl, 10 mM HEPES at pH 7.5 with an applied voltage of 100 mV. Traces were analysed using a custom Python CUSUM software to extract stepwise conductance increases.
Extended Data Fig. 5 I–V characteristics of individual carbon nanotube porins.
a. Schematics of the experimental set-up where a small area lipid bilayer with a single CNTP was formed over a SiNx nanopore fabricated on a silicon chip. An external voltage was applied across the bilayer to drive the ion transport through the CNTP channel. b. Current–voltage (I–V) curves recorded for an individual CNTP channel at different KCl concentrations, where the conductance obtained from the slopes of the linear fitting lines is 6.0 ± 0.7 pS at 5 mM KCl, 12.5 ± 3.5 pS at 20 mM KCl, 24.9 ± 5.8 pS at 50 mM KCl, and 35.8 ± 3.7 pS at 100 mM KCl. The conductance value recorded in a 100 mM KCl solution is similar to the value obtained in the CNTP insertion measurement.
Extended Data Fig. 6 Schematic of the multiscale model used to calculate the effective diffusion coefficient.
As discussed in the Methods section, the system is divided into 3 parts: (i) the bulk solution (highlighted in grey), (ii) the CNT entrance region (highlighted in purple), and (iii) the CNT interior (highlighted in yellow). The K+ ion diffusion in the CNT entrance and interior regions corresponds to anomalous subdiffusion, which is described using the generalized time-fractional diffusion equation with fractional diffusion coefficients and exponents.
Extended Data Fig. 7 Quantifying the role of entrance effects on the diffusion coefficient of K+ ions in the presence of the single-file water chain.
a. MSD of the K+ ion at the CNT entrance for the CNTP with 3 COO− groups. b. MSD of the K+ ion at the CNT entrance for the CNTP with 6 COO– groups. The MSD profiles in a and b have been obtained from 80 independent MD simulations, and are utilized in the multiscale model to calculate the effective diffusion coefficient, Deff, for CNTPs with 3 COO– and 6 COO– groups, respectively.
Extended Data Fig. 8 Formation of ion–water clusters during a 15 ns MD simulation run for the CNTP with 3 COO– groups.
a. Plot showing the variation of the number of water molecules inside the CNT, Nwater, during the first 7.5 ns of the MD simulation run in the presence of a 0.4 V·nm-1 external electric field applied along the z direction. Events corresponding to the translocation of K+ ions in the form of ion–water clusters are highlighted by the magenta solid boxes on the plot. The snapshots correspond to the time steps at which the K+ ions in the form of ion–water clusters cross the central region of the CNT (that is, z = 27 Å). b. Plot showing the variation of Nwater during the 7.5–15 ns MD simulation run. As shown in a and b, there are 5 distinct ion translocation events during the 15 ns MD simulation run, which are indicated by the magenta arrows.
Extended Data Fig. 9 Formation of ion–water clusters during a 15 ns MD simulation run for the CNTP with 6 COO– groups.
a. Plot showing the variation of the number of water molecules inside the CNT, Nwater during the first 7.5 ns of the MD simulation run. Similar to Extended Data Fig. 8, events corresponding to the translocation of K+ ions in the form of ion–water clusters are highlighted by the magenta solid boxes. b. Plot showing the variation of Nwater during the 7.5–15 ns MD simulation run. As shown in a and b, there are 10 distinct ion translocation events during the 15 ns MD simulation run, which are indicated by the magenta arrows.
Extended Data Fig. 10 Calculation of the electrophoretic mobilities and diffusion coefficients of ion–water clusters.
a. Variation of the z position of the K+ ion in cluster i (see Fig. 3e) as a function of the simulation time obtained in the presence of a 0.25 V·nm-1 electric field applied along the z direction. The simulation data (solid curve) is obtained from 5 independent MD simulations with different random velocities of the molecules in the system. The dashed curve represents the fit to the data obtained using the terminal velocity of the K+ ion, \(v_z = \frac{{dz}}{{dt}}\), which is estimated to be 24.7 ± 3.9 m·s-1. b. Variation of the z position of the K+ ion in cluster iv (see Fig. 3e) as a function of the simulation time obtained in the presence of a 0.25 V·nm-1 electric field applied along the z direction. Similar to a, the simulation data is obtained from 5 independent MD simulations. The calculated terminal velocity in this case is: vz = 54.7 ± 5.6 m·s-1. The terminal velocities obtained at three different electric fields have been used to obtain the data shown in Fig. 3g, h. c. Plot showing the variation of the MSD of cluster i in the absence of any electric field, that is, where Ez = 0. d. Plot showing the variation of the MSD of cluster iv in the absence of any electric field. Additional data on the variation of the terminal velocity and MSD of cluster v is presented in Supplementary Discussion S6.
Supplementary information
Supplementary Information
Supplementary Discussion 1–7, which covers: (1) the classical MD simulations carried out using all-atomistic polarizable force fields, (2) estimation of the number of COO– groups at the CNT entrance, (3) alignment of the CNT with respect to the lipid bilayer, (4) analysis of the ion-induced electronic polarization of the carbon atoms in the CNT, (5) calculation of the diffusion coefficient of K+ ions in the presence of the single-file water chain, (6) investigation of the electrophoretic transport of K+ ions; (7) experimental data for carbon nanotube porin characterization.
Supplementary Video 1
A video of a 2 ns duration all-atomistic MD simulation using polarizable force fields showing the formation of ion–water clusters inside a CNTP with 6 COO– groups in the presence of a 0.4 V nm−1 electric field applied along the z direction. The colour code used to represent the various atoms in the system is the same as that used in Fig. 3. The lipid bilayer surrounding the CNT is not shown for clarity.
Supplementary Video 2
A video of a 2 ns duration all-atomistic MD simulation using non-polarizable force fields showing the absence of any K+ ion translocation through the CNTP with 6 COO– groups in the presence of a 0.4 V nm−1 electric field applied along the z direction. All other settings are kept identical to those used to generate Supplementary Video 1. K+ ion translocation was also not observed for the CNTP with 3 COO– groups.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Misra, R.P., Li, Y. et al. Breakdown of the Nernst–Einstein relation in carbon nanotube porins. Nat. Nanotechnol. 18, 177–183 (2023). https://doi.org/10.1038/s41565-022-01276-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01276-0
This article is cited by
-
Nanofluidic logic with mechano–ionic memristive switches
Nature Electronics (2024)
-
When ions break the law
Nature Nanotechnology (2023)