Abstract
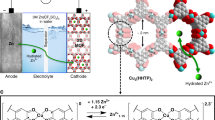
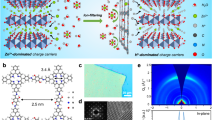
Ion exchange membranes are widely used to selectively transport ions in various electrochemical devices. Hydroxide exchange membranes (HEMs) are promising to couple with lower cost platinum-free electrocatalysts used in alkaline conditions, but are not stable enough in strong alkaline solutions. Herein, we present a Cu2+-crosslinked chitosan (chitosan-Cu) material as a stable and high-performance HEM. The Cu2+ ions are coordinated with the amino and hydroxyl groups of chitosan to crosslink the chitosan chains, forming hexagonal nanochannels (~1 nm in diameter) that can accommodate water diffusion and facilitate fast ion transport, with a high hydroxide conductivity of 67 mS cm−1 at room temperature. The Cu2+ coordination also enhances the mechanical strength of the membrane, reduces its permeability and, most importantly, improves its stability in alkaline solution (only 5% conductivity loss at 80 °C after 1,000 h). These advantages make chitosan-Cu an outstanding HEM, which we demonstrate in a direct methanol fuel cell that exhibits a high power density of 305 mW cm−2. The design principle of the chitosan-Cu HEM, in which ion transport channels are generated in the polymer through metal-crosslinking of polar functional groups, could inspire the synthesis of many ion exchange membranes for ion transport, ion sieving, ion filtration and more.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper and the Supplementary Information. Source data are provided with this paper. Other relevant data are available from the corresponding authors on reasonable request.
References
Varcoe, J. R. et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 7, 3135–3191 (2014).
Arges, C. G. & Zhang, L. Anion exchange membranes evolution toward high hydroxide ion conductivity and alkaline resiliency. ACS Appl. Energy Mater. 1, 2991–3012 (2018).
Lu, S., Pan, J., Huang, A., Zhuang, L. & Lu, J. Alkaline polymer electrolyte fuel cells completely free from noble metal catalysts. Proc. Natl Acad. Sci. USA 105, 20611–20614 (2008).
Xiong, P., Zhang, L., Chen, Y., Peng, S. & Yu, G. A chemistry and microstructure perspective on ion-conducting membranes for redox flow batteries. Angew. Chem. Int. Ed. Engl. 60, 2–31 (2021).
Kusoglu, A. & Weber, A. Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 117, 987–1104 (2017).
Gasteiger, H. A. & Marković, N. M. Just a dream—or future reality? Science 324, 48–49 (2009).
Jin, Z. et al. Understanding the inter-site distance effect in single-atom catalysts for oxygen electroreduction. Nat. Catal. 4, 615–622 (2021).
Hren, M., Božič, M., Fakin, D., Kleinschek, K. S. & Gorgieva, S. Alkaline membrane fuel cells: anion exchange membranes and fuels. Sustain. Energy Fuels 5, 604–637 (2021).
Setzler, B. P., Zhuang, Z., Wittkopf, J. A. & Yan, Y. Activity targets for nanostructured platinum-group-metal-free catalysts in hydroxide exchange membrane fuel cells. Nat. Nanotechnol. 11, 1020–1025 (2016).
Li, N., Zhang, Q., Wang, C., Lee, Y. M. & Guiver, M. D. Phenyltrimethylammonium functionalized polysulfone anion exchange membranes. Macromolecules 45, 2411–2419 (2012).
Kostalik, H. A. et al. Solvent processable tetraalkylammonium-functionalized polyethylene for use as an alkaline anion exchange membrane. Macromolecules 43, 7147–7150 (2010).
Hugar, K. M., Kostalik, H. A. & Coates, G. W. Imidazolium cations with exceptional alkaline stability: a systematic study of structure–stability relationships. J. Am. Chem. Soc. 137, 8730–8737 (2015).
Sata, T., Yamane, Y. & Matsusaki, K. Preparation and properties of anion exchange membranes having pyridinium or pyridinium derivatives as anion exchange groups. J. Polym. Sci. A Polym. Chem. 36, 49–58 (1998).
Sun, Z., Pan, J., Guo, J. & Yan, F. The alkaline stability of anion exchange membrane for fuel cell applications: the effects of alkaline media. Adv. Sci. 5, 1800065 (2018).
Wang, J., Gu, S., Kaspar, R. B., Zhang, B. & Yan, Y. Stabilizing the imidazolium cation in hydroxide-exchange membranes for fuel cells. ChemSusChem 6, 2079–2082 (2013).
Mustain, W. E., Chatenet, M., Page, M. & Kim, Y. S. Durability challenges of anion exchange membrane fuel cells. Energy Environ. Sci. 13, 2805–2838 (2020).
Noh, S., Jeon, J. Y., Adhikari, S., Kim, Y. S. & Bae, C. Molecular engineering of hydroxide conducting polymers for anion exchange membranes in electrochemical energy conversion technology. Acc. Chem. Res. 52, 2745–2755 (2019).
Kim, S.-K. Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activities and Applications 1st edn (CRC Press, 2011).
Xu, C., Nasrollahzadeh, M., Selva, M., Issaabadi, Z. & Luque, R. Waste-to-wealth: biowaste valorization into valuable bio(nano)materials. Chem. Soc. Rev. 48, 4791–4822 (2019).
Ogawa, K., Hirano, S., Miyanishi, T., Yui, T. & Watanabe, T. A new polymorph of chitosan. Macromolecules 17, 973–975 (1984).
Kraytsberg, A. & Ein-Eli, Y. Review of advanced materials for proton exchange membrane fuel cells. Energy Fuels 28, 7303–7330 (2014).
Peter, S. et al. Chitin and chitosan based composites for energy and environmental applications: a review. Waste Biomass Valoriz. 12, 4777–4804 (2020).
Sikorski, P., Hori, R. & Wada, M. Revisit of α-chitin crystal structure using high resolution X-ray diffraction data. Biomacromolecules 10, 1100–1105 (2009).
Okuyama, K. et al. Structural diversity of chitosan and its complexes. Carbohydr. Polym. 41, 237–247 (2000).
Ogawa, K., Oka, K. & Yui, T. X-ray study of chitosan-transition metal complexes. Chem. Mater. 5, 726–728 (1993).
Li, N., Guiver, M. D. & Binder, W. H. Towards high conductivity in anion-exchange membranes for alkaline fuel cells. ChemSusChem 6, 1376–1383 (2013).
Yassin, K., Rasin, I. G., Brandon, S. & Dekel, D. R. Quantifying the critical effect of water diffusivity in anion exchange membranes for fuel cell applications. J. Membr. Sci. 608, 118206 (2020).
Zelovich, T. et al. Hydroxide ion diffusion in anion-exchange membranes at low hydration: insights from ab initio molecular dynamics. Chem. Mater. 31, 5778–5787 (2019).
Zadok, I., Dekel, D. R. & Srebnik, S. Effect of ammonium cations on the diffusivity and structure of hydroxide ions in low hydration media. J. Phys. Chem. C 123, 27355–27362 (2019).
Tuckerman, M. E., Chandra, A. & Marx, D. Structure and dynamics of OH−(aq). Acc. Chem. Res. 39, 151–158 (2006).
Zadok, I. et al. Unexpected hydroxide ion structure and properties at low hydration. J. Mol. Lip. 313, 113485 (2020).
Zha, Y., Disabb-Miller, M. L., Johnson, Z. D., Hickner, M. A. & Tew, G. N. Metal-cation-based anion exchange membranes. J. Am. Chem. Soc. 134, 4493–4496 (2012).
Gu, S. et al. Permethyl cobaltocenium (Cp*2Co+) as an ultra-stable cation for polymer hydroxide-exchange membranes. Sci. Rep. 5, 11668 (2015).
Diesendruck, C. E. & Dekel, D. R. Water – a key parameter in the stability of anion exchange membrane fuel cells. Curr. Opin. Electrochem. 9, 173–178 (2018).
Dekel, D. R. et al. Effect of water on the stability of quaternary ammonium groups for anion exchange membrane fuel cell applications. Chem. Mater. 29, 4425–4431 (2017).
Gjineci, N., Aharonovich, S., Dekel, D. R. & Diesendruck, C. E. Increasing the alkaline stability of N,N-diaryl carbazolium salts using substituent electronic effects. ACS Appl. Mater. Interfaces 12, 49617–49625 (2020).
Dekel, D. R. et al. The critical relation between chemical stability of cations and water in anion exchange membrane fuel cells environment. J. Power Sources 375, 351–360 (2018).
Allen, F. I. et al. Morphology of hydrated as-cast Nafion revealed through cryo electron tomography. ACS Macro Lett. 4, 1–5 (2015).
Chen, N. et al. Cobaltocenium-containing polybenzimidazole polymers for alkaline anion exchange membrane applications. Polym. Chem. 8, 1381–1392 (2017).
Fan, J. et al. Cationic polyelectrolytes, stable in 10 M KOHaq at 100 °C. ACS Macro Lett. 6, 1089–1093 (2017).
Wang, J. et al. Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells. Nat. Energy 4, 392–398 (2019).
Liu, G. et al. Composite membranes from quaternized chitosan reinforced with surface-functionalized PVDF electrospun nanofibers for alkaline direct methanol fuel cells. J. Membr. Sci. 611, 118242 (2020).
Heinzel, A. & Barragán, V. M. A review of the state-of-the-art of the methanol crossover in direct methanol fuel cells. J. Power Sources 84, 70–74 (1999).
Zhu, H. et al. Anomalous scaling law of strength and toughness of cellulose nanopaper. Proc. Natl Acad. Sci. USA 112, 8971–8976 (2015).
Di Noto, V. et al. Inorganic–organic membranes based on Nafion, [(ZrO2)·(HfO2)0.25] and [(SiO2)·(HfO2)0.28] nanoparticles. Part II: relaxations and conductivity mechanism. Int. J. Hydrog. Energy 37, 6215–6227 (2012).
Kaspar, R. B. et al. Manipulating water in high-performance hydroxide exchange membrane fuel cells through asymmetric humidification and wetproofing. J. Electrochem. Soc. 162, F483–F488 (2015).
VandeVondele, J. & Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 127, 114105 (2007).
Goedecker, S., Teter, M. & Hutter, J. Separable dual-space Gaussian pseudopotentials. Phy. Rev. B 54, 1703–1710 (1996).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phy. Rev. A 38, 3098–3100 (1988).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Gillan, M. J., Alfè, D. & Michaelides, A. Perspective: how good is DFT for water? J. Chem. Phys. 144, 130901 (2016).
Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 52, 255–268 (1984).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Acknowledgements
L.H. and M.W. acknowledge support from the University of Maryland A. James Clark School of Engineering and Maryland Nanocenter, its Surface Analysis Center and AIMLab. R.M.B. and X.Z. acknowledge support from the US National Institute of Standards and Technology under cooperative agreement no. 70NANB15H261. The NMR work at Hunter College is supported by the US Office of Naval Research under grant no. N00014-20-1-2186. XAS research conducted at beamline 9-BM used resources of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. Research used resources of the Center for Functional Nanomaterials and the SMI beamline (12-ID) of the National Synchrotron Light Source II, both supported by US DOE Office of Science Facilities at Brookhaven National Laboratory under contract no. DE-SC0012704.
Author information
Authors and Affiliations
Contributions
L.H. and M.W. conceived the idea and designed the experiments. M.W. performed and interpreted the chitosan-Cu membrane fabrication, characterization and conductivity studies. X.Z., Y. Zhang and R.M.B. conducted and interpreted the aligned chitosan-Cu fabrication and X-ray diffraction measurements. Y. Zhao and Y.Y. performed the single fuel cell studies and the methanol permeability measurements. M.W. and S.J. took the digital photographs, zeta potential measurements and performed the tensile strain–stress tests. Q.W. and Y.Q. performed the AIMD and DFT simulations. S.B., M.G. and S.G.G. conducted the NMR measurements. T.W., N.L. and J.T.M. performed and interpreted the XAS measurements. M.W., X.Z., C.Y., A.B. and L.H. collectively wrote the paper. All authors commented on the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Peer review information
Nature Nanotechnology thanks Dario Dekel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–25, Tables 1–12 and Discussion.
Source data
Source Data Fig. 2
XPS and XAS spectra of chitosan-Cu.
Source Data Fig. 3
X-ray diffraction of chitosan-Cu membrane and aligned chitosan-Cu.
Source Data Fig. 4
EIS, conductivity evolution with relative humidity, stability of conductivity and simulated bond distance evolution.
Source Data Fig. 5
Methanol permeability, tensile stress–strain, single-cell polarization and power density, power density comparation, etc.
Rights and permissions
About this article
Cite this article
Wu, M., Zhang, X., Zhao, Y. et al. A high-performance hydroxide exchange membrane enabled by Cu2+-crosslinked chitosan. Nat. Nanotechnol. 17, 629–636 (2022). https://doi.org/10.1038/s41565-022-01112-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01112-5
This article is cited by
-
A Novel L-Cys@Cu MOF Embedding onto Cotton Fiber Surfaces to Exert Excellent Antiviral and Antibacterial Effects
Advanced Fiber Materials (2024)
-
Enhanced and synergistic catalytic activation by photoexcitation driven S−scheme heterojunction hydrogel interface electric field
Nature Communications (2023)
-
An in situ reduction strategy toward dendrite-free Zn anodes
Science China Materials (2023)
-
N and non-N site grafting piperidinium group to chitosan for anion exchange membrane
Ionics (2023)
-
An electrochemical sensor based on PEI/CS/GN composite–modified glassy carbon electrode for determination of Pb(II)
Ionics (2023)