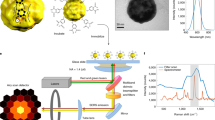
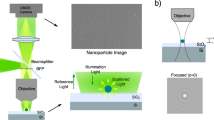
Abstract
Nanometric probes based on surface-enhanced Raman scattering (SERS) are promising candidates for all-optical environmental, biological and technological sensing applications with intrinsic quantitative molecular specificity. However, the effectiveness of SERS probes depends on a delicate trade-off between particle size, stability and brightness that has so far hindered their wide application in SERS imaging methodologies. In this Article, we introduce holographic Raman microscopy, which allows single-shot three-dimensional single-particle localization. We validate our approach by simultaneously performing Fourier transform Raman spectroscopy of individual SERS nanoparticles and Raman holography, using shearing interferometry to extract both the phase and the amplitude of wide-field Raman images and ultimately localize and track single SERS nanoparticles inside living cells in three dimensions. Our results represent a step towards multiplexed single-shot three-dimensional concentration mapping in many different scenarios, including live cell and tissue interrogation and complex anti-counterfeiting applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The materials and data that support the findings of this study are available from the corresponding authors on request.
Code availability
The software used for data analysis is available from the corresponding authors on request.
References
Albrecht, M. G. & Creighton, J. A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 4009, 5215–5217 (1977).
Jeanmaire, D. L. & Van Duyne, R. P. Surface Raman spectroelectrochemistry: Part 1. Heterocyclic, Aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 84, 1–20 (1977).
Fleischmann, M., Hendra, P. J. & McQuillan, A. J. Raman spectra of pyridine absorbed at a silver electrode. Chem. Phys. Lett. 26, 163–166 (1974).
Langer, J. et al. Present and future of surface-enhanced Raman scattering. ACS Nano 14, 28–117 (2020).
Lane, L. A., Qian, X. & Nie, S. SERS nanoparticles in medicine: from label-free detection to spectroscopic tagging. Chem. Rev. 115, 10489–10529 (2015).
Wang, Y., Yan, B. & Chen, L. SERS tags: novel optical nanoprobes for bioanalysis. Chem. Rev. 113, 1391–1428 (2013).
Zhang, X., Young, M. A., Lyandres, O. & Van Duyne, R. P. Rapid detection of an anthrax biomarker by surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 127, 4484–4489 (2005).
Grubisha, D. S., Lipert, R. J., Park, H.-Y., Driskell, J. & Porter, M. D. Femtomolar detection of prostate-specific antigen: an immunoassay based on surface-enhanced Raman scattering and immunogold labels. Anal. Chem. 75, 5936–5943 (2003).
Pazos, E. et al. Surface-enhanced Raman scattering surface selection rules for the proteomic liquid biopsy in real samples: efficient detection of the oncoprotein c-MYC. J. Am. Chem. Soc. 138, 14206–14209 (2016).
Li, J. F. et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 464, 392–395 (2010).
Dasary, S. S. R., Singh, A. K., Senapati, D., Yu, H. & Ray, P. C. Gold nanoparticle based label-free SERS probe for ultrasensitive and selective detection of trinitrotoluene. J. Am. Chem. Soc. 131, 13806–13812 (2009).
Pazos-Perez, N. et al. Ultrasensitive multiplex optical quantification of bacteria in large samples of biofluids. Sci. Rep. 6, 29014 (2016).
Pallaoro, A., Hoonejani, M. R., Braun, G. B., Meinhart, C. D. & Moskovits, M. Rapid identification by surface-enhanced Raman spectroscopy of cancer cells at low concentrations flowing in a microfluidic channel. ACS Nano 9, 4328–4336 (2015).
Palonpon, A. F. et al. Raman and SERS microscopy for molecular imaging of live cells. Nat. Protoc. 8, 677–692 (2013).
Rivera-Gil, P. et al. Plasmonic nanoprobes for real-time optical monitoring of nitric oxide inside living cells. Angew. Chem. Int. Ed. 125, 13939–13943 (2013).
Phan-Quang, G. C. et al. Three-dimensional surface-enhanced Raman scattering platforms: large-scale plasmonic hotspots for new applications in sensing, microreaction, and data storage. Acc. Chem. Res. 52, 1844–1854 (2019).
Jiang, W., Kim, B. Y. S., Rutka, J. T. & Chan, W. C. W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 3, 145–150 (2008).
Latychevskaia, T. & Fink, H.-W. Holographic time-resolved particle tracking by means of three-dimensional volumetric deconvolution. Opt. Express 22, 20994–21003 (2014).
Molaei, M. & Sheng, J. Imaging bacterial 3D motion using digital in-line holographic microscopy and correlation-based de-noising algorithm. Opt. Express 22, 32119–32137 (2014).
Memmolo, P. et al. Recent advances in holographic 3D particle tracking. Adv. Opt. Photonics 7, 713–755 (2015).
Gabor, D. A new microscopic principle. Nature 161, 777–778 (1948).
Cuche, E., Marquet, P. & Depeursinge, C. Spatial filtering for zero-order and twin-image elimination in digital off-axis holography. Appl. Opt. 39, 4070–4075 (2000).
Bon, P. et al. Self-interference 3D super-resolution microscopy for deep tissue investigations. Nat. Methods 15, 449–454 (2018).
Patorski, K. The self-imaging phenomenon and its applications. Prog. Opt. 27, 1–108 (1989).
Bon, P., Maucort, G., Wattellier, B. & Monneret, S. Quadriwave lateral shearing interferometry for quantitative phase microscopy of living cells. Opt. Express 17, 13080–13094 (2009).
Camden, J. P. et al. Probing the structure of single-molecule surface-enhanced Raman scattering hot spots. J. Am. Chem. Soc. 130, 12616–12617 (2008).
Michaels, A. M., Jiang, J. & Brus, L. Ag nanocrystal junctions as the site for surface-enhanced Raman scattering of single rhodamine 6G molecules. J. Phys. Chem. B 104, 11965–11971 (2000).
Maznev, A. A., Crimmins, T. F. & Nelson, K. A. How to make femtosecond pulses overlap. Opt. Lett. 23, 1378–1380 (1998).
Takeda, M., Ina, H. & Kobayashi, S. Fourier-transform method of fringe-pattern analysis for computer-based topography and interferometry. J. Opt. Soc. Am. 72, 156–160 (1982).
Arnison, M. R., Larkin, K. G., Sheppard, C. J. R., Smith, N. I. & Cogswell, C. J. Linear phase imaging using differential interference contrast microscopy. J. Microsc. 214, 7–12 (2003).
Choi, I., Lee, K. & Park, Y. Compensation of aberration in quantitative phase imaging using lateral shifting and spiral phase integration. Opt. Express 25, 30771–30779 (2017).
Jaqaman, K. et al. Robust single-particle tracking in live-cell time-lapse sequences. Nat. Methods 5, 695–702 (2008).
Rubin, M., Dardikman, G., Mirsky, S. K., Turko, N. A. & Shaked, N. T. Six-pack off-axis holography. Opt. Lett. 42, 4611–4614 (2017).
Acknowledgements
We thank M. Rivas for her support with the live-cell experiments. We acknowledge support by the Ministry of Science, Innovation and Universities (MCIU/AEI: RTI2018-099957-J-I00 and PGC2018-096875-B-I00), the Ministry of Economy (MINECO: CTQ2017-88648-R, RYC-2015-19107 and ‘Severo Ochoa’ programme for Centers of Excellence in R&D CEX2019-000910-S), the Catalan AGAUR (2017SGR1369 and 2017SGR883), Fundació Privada Cellex, Fundació Privada Mir-Puig, the Generalitat de Catalunya through the CERCA programme and the Universitat Rovira i Virgili (FR 2019-B2). N.F.v.H. acknowledges the financial support by the European Commission (ERC Advanced Grant 670949-LightNet).
Author information
Authors and Affiliations
Contributions
M.L. constructed the optical experiment, performed the measurements and analysed the data. M.L. conceived the experiment. N.P.P. and R.A.A.P. synthesized and characterized the nanoparticles. M.L. wrote the manuscript. M.L., N.F.v.H. and R.A.A.P. contributed to the interpretation of the data, discussion and writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Pasquale Memmolo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8 and Information S1–S7.
Supplementary Video 1
Live-cell tracking video supporting Fig. 6, showing bright-field (grey) and SERS (pink) signals.
Rights and permissions
About this article
Cite this article
Liebel, M., Pazos-Perez, N., van Hulst, N.F. et al. Surface-enhanced Raman scattering holography. Nat. Nanotechnol. 15, 1005–1011 (2020). https://doi.org/10.1038/s41565-020-0771-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-0771-9
This article is cited by
-
Ultrafast charge transfer in mixed-dimensional WO3-x nanowire/WSe2 heterostructures for attomolar-level molecular sensing
Nature Communications (2023)
-
Combining external attention GAN with deep convolutional neural networks for real–fake identification of luxury handbags
The Visual Computer (2023)
-
Comprehensive deep learning model for 3D color holography
Scientific Reports (2022)
-
Roadmap of incoherent digital holography
Applied Physics B (2022)