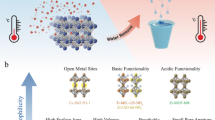
Abstract
The advancement of additional methods for freshwater generation is imperative to effectively address the global water shortage crisis. In this regard, extraction of the ubiquitous atmospheric moisture is a powerful strategy allowing for decentralized access to potable water. The energy requirements as well as the temporal and spatial restrictions of this approach can be substantially reduced if an appropriate sorbent is integrated in the atmospheric water generator. Recently, metal–organic frameworks (MOFs) have been successfully employed as sorbents to harvest water from air, making atmospheric water generation viable even in desert environments. Herein, the latest progress in the development of MOFs capable of extracting water from air and the design of atmospheric water harvesters deploying such MOFs are reviewed. Furthermore, future directions for this emerging field, encompassing both material and device improvements, are outlined.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mekonnen, M. M. & Hoekstra, A. Y. Four billion people facing severe water scarcity. Sci. Adv. 2, e1500323 (2016).
The Sustainable Development Goals Report 2018 (United Nations, 2018).
Oelkers, E. H., Hering, J. G. & Zhu, C. Water: Is there a global crisis? Elements 7, 157–162 (2011).
WWAP (UNESCO World Water Assessment Programme). The United Nations World Water Development Report 2019: Leaving No One Behind (UNESCO, 2019).
Elimelech, M. & Phillip, W. A. The future of seawater desalination: Energy, technology, and the environment. Science 333, 712–717 (2011).
Wahlgren, R. V. Atmospheric water vapour processor designs for potable water production: A review. Water Res. 35, 1–22 (2001).
Klemm, O. et al. Fog as a fresh-water resource: Overview and perspectives. Ambio 41, 221–234 (2012).
Bagheri, F. Performance investigation of atmospheric water harvesting systems. Water Resour. Ind. 20, 23–28 (2018).
Gido, B., Friedler, E. & Broday, D. M. Assessment of atmospheric moisture harvesting by direct cooling. Atmos. Res. 182, 156–162 (2016).
Elmer, T. H. & Franklin Hyde, J. Recovery of water from atmospheric air in arid climates. Sep. Sci. Technol. 21, 251–266 (1986).
Furukawa, H. et al. Water adsorption in porous metal-organic frameworks and related materials. J. Am. Chem. Soc. 136, 4369–4381 (2014).
Yu, N., Wang, R. Z., Lu, Z. S. & Wang, L. W. Development and characterization of silica gel-LiCl composite sorbents for thermal energy storage. Chem. Eng. Sci. 111, 73–84 (2014).
Ng, E.-P. & Mintova, S. Nanoporous materials with enhanced hydrophilicity and high water sorption capacity. Microporous Mesoporous Mater. 114, 1–26 (2008).
Krajnc, A. et al. Superior performance of microporous aluminophosphate with LTA topology in solar-energy storage and heat reallocation. Adv. Energy Mater. 7, 1601815 (2017).
Yaghi, O. M. et al. Reticular synthesis and the design of new materials. Nature 423, 705–714 (2003).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal-organic frameworks. Science 341, 1230444 (2013).
Kalmutzki, M. J., Hanikel, N. & Yaghi, O. M. Secondary building units as the turning point in the development of the reticular chemistry of MOFs. Sci. Adv. 4, eaat9180 (2018).
Furukawa, H. et al. Ultrahigh porosity in metal-organic frameworks. Science 329, 424–428 (2010).
Farha, O. K. et al. Metal-organic framework materials with ultrahigh surface areas: Is the sky the limit? J. Am. Chem. Soc. 134, 15016–15021 (2012).
Hönicke, I. M. et al. Balancing mechanical stability and ultrahigh porosity in crystalline framework materials. Angew. Chem. Int. Ed. 57, 13780–13783 (2018).
Kim, H. et al. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 356, 430–434 (2017).
Kim, H. et al. Adsorption-based atmospheric water harvesting device for arid climates. Nat. Commun. 9, 1191 (2018).
Fathieh, F. et al. Practical water production from desert air. Sci. Adv. 4, eaat3198 (2018).
Hanikel, N. et al. Rapid cycling and exceptional yield in a metal-organic framework water harvester. ACS Cent. Sci. 5, 1699–1706 (2019).
Burtch, N. C., Jasuja, H. & Walton, K. S. Water stability and adsorption in metal-organic frameworks. Chem. Rev. 114, 10575–10612 (2014).
Kalmutzki, M. J., Diercks, C. S. & Yaghi, O. M. Metal-organic frameworks for water harvesting from air. Adv. Mater. 30, 1704304 (2018).
Rieth, A. J., Wright, A. M. & Dincă, M. Kinetic stability of metal-organic frameworks for corrosive and coordinating gas capture. Nat. Rev. Mater. 4, 708–725 (2019).
Choi, H. J., Dincǎ, M., Dailly, A. & Long, J. R. Hydrogen storage in water-stable metal-organic frameworks incorporating 1,3- and 1,4-benzenedipyrazolate. Energy Environ. Sci. 3, 117–123 (2010).
Jasuja, H. & Walton, K. S. Effect of catenation and basicity of pillared ligands on the water stability of MOFs. Dalt. Trans. 42, 15421–15426 (2013).
Chen, Z. et al. Reticular access to highly porous acs-MOFs with rigid trigonal prismatic linkers for water sorption. J. Am. Chem. Soc. 141, 2900–2905 (2019).
Mondloch, J. E. et al. Are Zr6-based MOFs water stable? Linker hydrolysis vs. capillary-force-driven channel collapse. Chem. Commun. 50, 8944–8946 (2014).
Wahiduzzaman, M., Lenzen, D., Maurin, G., Stock, N. & Wharmby, M. T. Rietveld refinement of MIL-160 and its structural flexibility upon H2O and N2 adsorption. Eur. J. Inorg. Chem. 2018, 3626–3632 (2018).
Eddaoudi, M. et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295, 469–472 (2002).
Cavka, J. H., Olsbye, U., Guillou, N., Bordiga, S. & Lillerud, K. P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 130, 13850–13851 (2008).
Abtab, S. M. T. et al. Reticular chemistry in action: A hydrolytically stable MOF capturing twice its weight in adsorbed water. Chem 4, 94–105 (2018).
Akiyama, G. et al. Effect of functional groups in MIL-101 on water sorption behavior. Microporous Mesoporous Mater. 157, 89–93 (2012).
Reinsch, H. et al. Structures, sorption characteristics, and nonlinear optical properties of a new series of highly stable aluminum MOFs. Chem. Mater. 25, 17–26 (2013).
Cadiau, A. et al. Design of hydrophilic metal organic framework water adsorbents for heat reallocation. Adv. Mater. 27, 4775–4780 (2015).
Brozek, C. K. & Dincă, M. Cation exchange at the secondary building units of metal-organic frameworks. Chem. Soc. Rev. 43, 5456–5467 (2014).
Wright, A. M., Rieth, A. J., Yang, S., Wang, E. N. & Dincǎ, M. Precise control of pore hydrophilicity enabled by post-synthetic cation exchange in metal-organic frameworks. Chem. Sci. 9, 3856–3859 (2018).
Rieth, A. J. et al. Record-setting sorbents for reversible water uptake by systematic anion-exchanges in metal-organic frameworks. J. Am. Chem. Soc. 141, 13858–13866 (2019).
Rubio-Martinez, M. et al. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 46, 3453–3480 (2017).
Lenzen, D. et al. Scalable green synthesis and full-scale test of the metal-organic framework CAU-10-H for use in adsorption-driven chillers. Adv. Mater. 30, 1705869 (2017).
Reinsch, H., Waitschat, S., Chavan, S. M., Lillerud, K. P. & Stock, N. A facile “green” route for scalable batch production and continuous synthesis of zirconium MOFs. Eur. J. Inorg. Chem. 2016, 4490–4498 (2016).
Julien, P. A., Mottillo, C. & Friščić, T. Metal-organic frameworks meet scalable and sustainable synthesis. Green Chem. 19, 2729–2747 (2017).
Ghosh, P., Colón, Y. J. & Snurr, R. Q. Water adsorption in UiO-66: The importance of defects. Chem. Commun. 50, 11329–11331 (2014).
Hossain, M. I. & Glover, T. G. Kinetics of water adsorption in UiO-66 MOF. Ind. Eng. Chem. Res. 58, 10550–10558 (2019).
Huang, B. L., McGaughey, A. J. H. & Kaviany, M. Thermal conductivity of metal-organic framework 5 (MOF-5): Part I. Molecular dynamics simulations. Int. J. Heat Mass Transf. 50, 393–404 (2007).
Huang, B. L. et al. Thermal conductivity of a metal-organic framework (MOF-5): Part II. Measurement. Int. J. Heat Mass Transf. 50, 405–411 (2007).
Yang, S., Kim, H., Narayanan, S., McKay, I. S. & Wang, E. N. Dimensionality effects of carbon-based thermal additives for microporous adsorbents. Mater. Des. 85, 520–526 (2015).
Ming, Y. et al. Anisotropic thermal transport in MOF-5 composites. Int. J. Heat Mass Transf. 82, 250–258 (2015).
Yang, S., Huang, X., Chen, G. & Wang, E. N. Three-dimensional graphene enhanced heat conduction of porous crystals. J. Porous Mater. 23, 1647–1652 (2016).
Chiavazzo, E., Morciano, M., Viglino, F., Fasano, M. & Asinari, P. Passive solar high-yield seawater desalination by modular and low-cost distillation. Nat. Sustain. 1, 763–772 (2018).
González-Bravo, R., Ponce-Ortega, J. M. & El-Halwagi, M. M. Optimal design of water desalination systems involving waste heat recovery. Ind. Eng. Chem. Res. 56, 1834–1847 (2017).
Wang, Z. et al. Pathways and challenges for efficient solar-thermal desalination. Sci. Adv. 5, eaax0763 (2019).
Cho, H. J., Preston, D. J., Zhu, Y. & Wang, E. N. Nanoengineered materials for liquid-vapour phase-change heat transfer. Nat. Rev. Mater. 2, 16092 (2016).
Ahlers, M., Buck-Emden, A. & Bart, H.-J. Is dropwise condensation feasible? A review on surface modifications for continuous dropwise condensation and a profitability analysis. J. Adv. Res. 16, 1–13 (2019).
Warsinger, D. M., Mistry, K. H., Nayar, K. G., Chung, H. W. & Lienhard, V. J. H. Entropy generation of desalination powered by variable temperature waste heat. Entropy 17, 7530–7566 (2015).
Brogioli, D., La Mantia, F. & Yip, N. Y. Thermodynamic analysis and energy efficiency of thermal desalination processes. Desalination 428, 29–39 (2018).
Acker, J. G. & Leptoukh, G. Online analysis enhances use of NASA Earth Science Data. Eos 88, 14–17 (2007).
Acknowledgements
Financial support for this research is provided by the King Abdulaziz City for Science and Technology as part of a joint KACST-UC Berkeley collaboration (Center of Excellence for Nanomaterials and Clean Energy Applications). N.H. thanks the Studienstiftung des deutschen Volkes and acknowledges the receipt of the Kavli ENSI Philomathia Graduate Fellowship. M.S.P. is grateful to the Swiss National Science Foundation for funding through the Early Postdoc Mobility fellowship program (award P2ELP2_175067). We thank C. Gropp for useful discussions. Analyses and visualizations used in this Review Article were produced with the Giovanni online data system, developed and maintained by the NASA GES DISC. We also acknowledge the AIRS mission scientists and associated NASA personnel for the production of the data used for the MHI calculations in this publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
O.M.Y. is co-founder of Water Harvesting Inc., aiming at commercializing related technologies.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hanikel, N., Prévot, M.S. & Yaghi, O.M. MOF water harvesters. Nat. Nanotechnol. 15, 348–355 (2020). https://doi.org/10.1038/s41565-020-0673-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-0673-x
This article is cited by
-
Roll-to-roll fabrication of large-area metal–organic framework-based membranes for high-performance aqueous separations
Nature Water (2024)
-
Sustainable moisture energy
Nature Reviews Materials (2024)
-
A 5,6,11,12,17,18-Hexaazatrinaphthylene-Based Luminescent Metal-Organic Framework as Ornidazole Sensor with Extremely High Sensitivity
Journal of Cluster Science (2024)
-
Mixed-linker strategy for suppressing structural flexibility of metal-organic framework membranes for gas separation
Communications Chemistry (2023)
-
Investigating the mechanical stability of flexible metal–organic frameworks
Communications Chemistry (2023)