Abstract
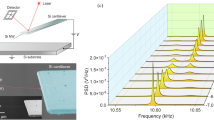
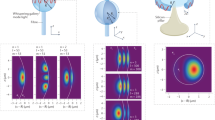
Low-frequency vibration modes of biological particles, such as proteins, viruses and bacteria, involve coherent collective vibrations at frequencies in the terahertz and gigahertz domains. These vibration modes carry information on their structure and mechanical properties, which are good indicators of their biological state. In this work, we harnessed a particular regime in the physics of coupled mechanical resonators to directly measure these low-frequency mechanical resonances of a single bacterium. We deposit the bacterium on the surface of an ultrahigh frequency optomechanical disk resonator in ambient conditions. The vibration modes of the disk and bacterium hybridize when their associated frequencies are similar. We developed a general theoretical framework to describe this coupling, which allows us to retrieve the eigenfrequencies and mechanical loss of the bacterium low-frequency vibration modes (quality factor). Additionally, we analysed the effect of hydration on these vibrational modes. This work demonstrates that ultrahigh frequency optomechanical resonators can be used for vibrational spectrometry with the unique capability to obtain information on single biological entities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request
Change history
29 April 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41565-020-0698-1
References
Smith, E. & Dent, G. Modern Raman Spectroscopy: A Practical Approach (Wiley, 2019).
Davydov, A. S. The theory of contraction of proteins under their excitation. J. Theor. Biol. 38, 559–569 (1973).
Hay, S. & Scrutton, N. S. Good vibrations in enzyme-catalysed reactions. Nat. Chem. 4, 161–168 (2012).
Ford, L. H. Estimate of the vibrational frequencies of spherical virus particles. Phys. Rev. E 67, 051924 (2003).
Sirotkin, S., Mermet, A., Bergoin, M., Ward, V. & Van Etten, J. L. Viruses as nanoparticles: structure versus collective dynamics. Phys. Rev. E 90, 022718 (2014).
Zinin, P., Allen, J. S. III & Levin, V. Mechanical resonances of bacteria cells. Phys. Rev. E 72, 061907 (2005).
Turton, D. A. et al. Terahertz underdamped vibrational motion governs protein–ligand binding in solution. Nat. Commun. 5, 3999 (2014).
Acbas, G., Niessen, K. A., Snell, E. H. & Markelz, A. G. Optical measurements of long-range protein vibrations. Nat. Commun. 5, 3076 (2014).
Longo, G. et al. Antibiotic-induced modifications of the stiffness of bacterial membranes. J. Microbiol. Methods 93, 80–84 (2013).
Köhler, J. et al. Mutation of the myosin converter domain alters cross-bridge elasticity. Proc. Natl Acad. Sci. USA 99, 3557–3562 (2002).
Greber, U. F. Virus and host mechanics support membrane penetration and cell entry. J. Virol. 90, 3802–3805 (2016).
Roos, W. H., Bruinsma, R. & Wuite, G. J. L. Physical virology. Nat. Phys. 6, 733–743 (2010).
Malvar, O. et al. Mass and stiffness spectrometry of nanoparticles and whole intact bacteria by multimode nanomechanical resonators. Nat. Commun. 7, 13452 (2016).
Dexheimer, S. L. Terahertz Spectroscopy: Principles and Applications (CRC, 2007).
Stephanidis, B., Adichtchev, S., Gouet, P., McPherson, A. & Mermet, A. Elastic properties of viruses. Biophys. J. 93, 1354–1359 (2007).
Zinin, P. V. & Allen, J. S. III Deformation of biological cells in the acoustic field of an oscillating bubble. Phys. Rev. E 79, 021910 (2009).
Favero, I. & Karrai, K. Optomechanics of deformable optical cavities. Nat. Photon. 3, 201–205 (2009).
Ding, L. et al. High frequency GaAs nano-optomechanical disk resonator. Phys. Rev. Lett. 105, 263903 (2010).
Gil-Santos, E. et al. High-frequency nano-optomechanical disk resonators in liquids. Nat. Nanotechnol. 10, 810–816 (2015).
Baker, C. et al. Photoelastic coupling in gallium arsenide optomechanical disk resonators. Opt. Express 22, 14072–14086 (2014).
Hanay, M. S. et al. Single-protein nanomechanical mass spectrometry in real time. Nat. Nanotechnol. 7, 602–608 (2012).
Dominguez-Medina, S. et al. Neutral mass spectrometry of virus capsids above 100 megadaltons with nanomechanical resonators. Science 362, 918–922 (2018).
Liu, F., Alaie, S., Leseman, Z. C. & Hossein-Zadeh, M. Sub-pg mass sensing and measurement with an optomechanical oscillator. Opt. Express 21, 19555–19567 (2013).
Kosaka, P. M., Calleja, M. & Tamayo, J. Optomechanical devices for deep plasma cancer proteomics. Semin. Cancer Biol. 52, 26–38 (2018).
Spletzer, M., Raman, A., Wu, A. Q., Xu, X. & Reifenberger, R. Ultrasensitive mass sensing using mode localization in coupled microcantilevers. Appl. Phys. Lett. 88, 254102 (2006).
Gil-Santos, E. et al. Mass sensing based on deterministic and stochastic responses of elastically coupled nanocantilevers. Nano Lett. 9, 4122–4127 (2009).
Gil-Santos, E., Ramos, D., Pini, V., Calleja, M. & Tamayo, J. Exponential tuning of the coupling constant of coupled microcantilevers by modifying their separation. Appl. Phys. Lett. 98, 123108 (2011).
Stassi, S. et al. Large-scale parallelization of nanomechanical mass spectrometry with weakly-coupled resonators. Nat. Commun. 10, 3647 (2019).
Duval, E. Far-infrared and Raman vibrational transitions of a solid sphere: selection rules. Phys. Rev. B 46, 5795–5797 (1992).
Ruz, J. J., Tamayo, J., Pini, V., Kosaka, P. M. & Calleja, M. Physics of nanomechanical spectrometry of viruses. Sci. Rep. 4, 6051 (2014).
Furusawa, H., Sekine, T. & Ozeki, T. Hydration and viscoelastic properties of high-and low-density polymer brushes using a quartz-crystal microbalance based on admittance analysis (QCM-A). Macromolecules 49, 3463–3470 (2016).
Domínguez, C. M. et al. Effect of water–DNA interactions on elastic properties of DNA self-assembled monolayers. Sci. Rep. 7, 536 (2017).
Bateman, J. B., Stevens, C. L., Mercer, W. B. & Carstensen, E. L. Relative humidity and the killing of bacteria: the variation of cellular water content with external relative humidity or osmolality. Microbiology 29, 207–219 (1962).
Rubel, G. O. Measurement of water vapor sorption by single biological aerosols. Aerosol Sci. Tech. 27, 481–490 (1997).
Deng, Y., Sun, M. & Shaevitz, J. W. Direct measurement of cell wall stress stiffening and turgor pressure in live bacterial cells. Phys. Rev. Lett. 107, 158101 (2011).
Nikiyan, H., Vasilchenko, A. & Deryabin, D. Humidity-dependent bacterial cells functional morphometry investigations using atomic force microscope. Int. J. Microbiol. 2010, 704170 (2010).
Thwaites, J. J. & Surana, U. C. Mechanical properties of Bacillus subtilis cell walls: effects of removing residual culture medium. J. Bacteriol. 173, 197–203 (1991).
Kulasinski, K., Guyer, R., Keten, S., Derome, D. & Carmeliet, J. Impact of moisture adsorption on structure and physical properties of amorphous biopolymers. Macromolecules 48, 2793–2800 (2015).
Guillet, Y., Abbas, A., Ravaine, S. & Audoin, B. Ultrafast microscopy of the vibrational landscape of a single nanoparticle. Appl. Phys. Lett. 114, 091904 (2019).
Hsueh, C.-C., Gordon, R. & Rottler, J. Dewetting during terahertz vibrations of nanoparticles. Nano Lett. 18, 773–777 (2018).
Acknowledgements
This work was supported by the European Union’s Horizon 2020 research and innovation program under grant agreement no. 731868 – VIRUSCAN and European Research Council grants 681275 – LIQUIDMASS- ERC- CoG-2015 and 770933-NOMLI-ERC-CoG 2017, by the Spanish Science, Innovation and Universities Ministry through project CELLTANGLE reference RTI2018-099369-B-I00 and Ramón y Cajal grant RYC-2017-21640 to P.M.K. and by the Comunidad de Madrid (iLUNG B2017/BMD-3884) with support from the EU (FEDER, FSE). We acknowledge J. Mingorance for guidance and for providing the bacterial samples. All the authors acknowledge service from the IMN X-SEM Laboratory, which is funded by MCIU (project CSIC13-4E-1794) and the EU (FEDER, FSE). E.G.S. acknowledges financial support by the Fundación General CSIC (Programa ComFuturo), as well as Marie-Sklodowska Curie Actions (H2020-MSCA-IF-2015) under the NOMBIS project (703354).
Author information
Authors and Affiliations
Contributions
E.G.-S., M.C. and J.T. conceived and designed the experiments, E.G.-S. and O.M. performed the experiments, E.G.-S., J.J.R. and J.T. analysed the data and developed the theory, S.G.-L., O.M. and P.M.K. contributed materials and tools to deposit the bacteria, E.G.-S., A.L. and I.F. designed and fabricated the devices and J.T., M.C. and E.G.-S. co-wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
E.G.-S., J.J.R., O.M., M.C. and J.T. are inventors of a related patent (WO/2019/229000) owned by their host institution Consejo Superior de Investigaciones Científicas.
Additional information
Peer review information Nature Nanotechnology thanks Carlo Ricciardi, Yun-Feng Xiao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Materials and Methods, Figs. 1–4 and refs. 1–7.
Rights and permissions
About this article
Cite this article
Gil-Santos, E., Ruz, J.J., Malvar, O. et al. Optomechanical detection of vibration modes of a single bacterium. Nat. Nanotechnol. 15, 469–474 (2020). https://doi.org/10.1038/s41565-020-0672-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-0672-y
This article is cited by
-
Metamaterial-based passive analog processor for wireless vibration sensing
Communications Engineering (2024)
-
Nonlinear interactions between vibration modes with vastly different eigenfrequencies
Communications Physics (2023)
-
Single-particle photoacoustic vibrational spectroscopy using optical microresonators
Nature Photonics (2023)
-
New opto-electro-mechanical sensor for two-dimensions dosimetry based on radiochromic films
Scientific Reports (2023)
-
Listening to microorganisms with light
Nature Photonics (2023)