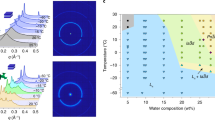
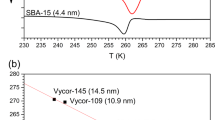
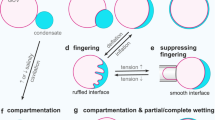
Abstract
Water is a ubiquitous liquid with unique physicochemical properties, whose nature has shaped our planet and life as we know it. Water in restricted geometries has different properties than in bulk. Confinement can prevent low-temperature crystallization of the molecules into a hexagonal structure and thus create a state of amorphous water. To understand the survival of life at subzero temperatures, it is essential to elucidate this behaviour in the presence of nanoconfining lipidic membranes. Here we introduce a family of synthetic lipids with designed cyclopropyl modifications in the hydrophobic chains that exhibit unique liquid-crystalline behaviour at low temperature, which enables the maintenance of amorphous water down to ~10 K due to nanoconfinement. The combination of experiments and molecular dynamics simulations unveils a complex lipid–water phase diagram in which bicontinuous cubic and lamellar liquid crystalline phases that contain subzero liquid, glassy or ice water emerge as a competition between the two components, each pushing towards its thermodynamically favoured state.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Chakraborty, S., Kumar, H., Dasgupta, C. & Maiti, P. K. Confined water: structure, dynamics, and thermodynamics. Acc. Chem. Res. 50, 2139–2146 (2017).
Kumar, P., Buldyrev, S. V., Starr, F. W., Giovambattista, N. & Stanley, H. E. Thermodynamics, structure, and dynamics of water confined between hydrophobic plates. Phys. Rev. E 72, 051503 (2005).
Levinger, N. E. Water in confinement. Science 298, 1722–1723 (2002).
Holt, J. K. et al. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 312, 1034–1037 (2006).
Chiavazzo, E., Fasano, M., Asinari, P. & Decuzzi, P. Scaling behaviour for the water transport in nanoconfined geometries. Nat. Commun. 5, 4565 (2014).
Hande, V. R. & Chakrabarty, S. Exploration of the presence of bulk-like water in AOT reverse micelles and water-in-oil nanodroplets: the role of charged interfaces, confinement size and properties of water. Phys. Chem. Chem. Phys. 18, 21767–21779 (2016).
Moilanen, D. E., Levinger, N. E., Spry, D. B. & Fayer, M. D. Confinement or the nature of the interface? Dynamics of nanoscopic water. J. Am. Chem. Soc. 129, 14311–14318 (2007).
Findenegg, G. H., Jahnert, S., Akcakayiran, D. & Schreiber, A. Freezing and melting of water confined in silica nanopores. Chem. Phys. Chem. 9, 2651–2659 (2008).
Cerveny, S., Mallamace, F., Swenson, J., Vogel, M. & Xu, L. M. Confined water as model of supercooled water. Chem. Rev. 116, 7608–7625 (2016).
Bergman, R. & Swenson, J. Dynamics of supercooled water in confined geometry. Nature 403, 283–286 (2000).
Venables, D. S., Huang, K. & Schmuttenmaer, C. A. Effect of reverse micelle size on the librational band of confined water and methanol. J. Phys. Chem. B 105, 9132–9138 (2001).
Swenson, J., Kargl, F., Berntsen, P. & Svanberg, C. Solvent and lipid dynamics of hydrated lipid bilayers by incoherent quasielastic neutron scattering. J. Chem. Phys. 129, 045101 (2008).
Miskowiec, A. et al. On the structure and dynamics of water associated with single-supported zwitterionic and anionic membranes. J. Chem. Phys. 146, 125102 (2017).
Boetius, A., Anesio, A. M., Deming, J. W., Mikucki, J. A. & Rapp, J. Z. Microbial ecology of the cryosphere: sea ice and glacial habitats. Nat. Rev. Microbiol. 13, 677–690 (2015).
Kim, J. et al. Ultrafast hydration dynamics in the lipidic cubic phase: discrete water structures in nanochannels. J. Phys. Chem. B 110, 21994–22000 (2006).
Wachter, W., Trimmel, G., Buchner, R. & Glatter, O. Dynamics of water confined in self-assembled monoglyceride–water–oil phases. Soft Matter 7, 1409–1417 (2011).
Qiu, H. & Caffrey, M. The phase diagram of the monoolein/water system: metastability and equilibrium aspects. Biomaterials 21, 223–234 (2000).
Kulkarni, C. V. et al. Engineering bicontinuous cubic structures at the nanoscale—the role of chain splay. Soft Matter 6, 3191–3194 (2010).
Hyde, S. T. Bicontinuous structures in lyotropic liquid crystals and crystalline hyperbolic surfaces. Curr. Opin. Solid State Mater. Sci. 1, 653–662 (1996).
Seddon, J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase-transitions of lipids. Biochim. Biophys. Acta 1031, 1–69 (1990).
Kulkarni, C. V., Wachter, W., Iglesias-Salto, G., Engelskirchen, S. & Ahualli, S. Monoolein: a magic lipid? Phys. Chem. Chem. Phys. 13, 3004–3021 (2011).
Fong, W. K., Negrini, R., Vallooran, J. J., Mezzenga, R. & Boyd, B. J. Responsive self-assembled nanostructured lipid systems for drug delivery and diagnostics. J. Colloid Interface Sci. 484, 320–339 (2016).
Duss, M. et al. Lipidic mesophases as novel nanoreactor scaffolds for organocatalysts: heterogeneously catalyzed asymmetric aldol reactions in confined water. ACS Appl. Mater. Interfaces 10, 5114–5124 (2018).
Speziale, C., Salvati Manni, L., Manatschal, C., Landau, E. M. & Mezzenga, R. A macroscopic H+ and Cl– ions pump via reconstitution of EcClC membrane proteins in lipidic cubic mesophases. Proc. Natl Acad. Sci. USA 113, 7491–7496 (2016).
Landau, E. M. & Rosenbusch, J. P. Lipidic cubic phases: a novel concept for the crystallization of membrane proteins. Proc. Natl Acad. Sci. USA 93, 14532–14535 (1996).
Cherezov, V. et al. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318, 1258–1265 (2007).
Luzzati, V. & Husson, F. The structure of the liquid-crystalline phasis of lipid–water systems. J. Cell Biol. 12, 207–219 (1962).
Salvati Manni, L. et al. Phase behavior of a designed cyclopropyl analogue of monoolein: implications for low-temperature membrane protein crystallization. Angew. Chem. Int. Ed. 54, 1027–1031 (2015).
Rao, V., Fujiwara, N., Porcelli, S. A. & Glickman, M. S. Mycobacterium tuberculosis controls host innate immune activation through cyclopropane modification of a glycolipid effector molecule. J. Exp. Med. 201, 535–543 (2005).
Fong, C., Le, T. & Drummond, C. J. Lyotropic liquid crystal engineering-ordered nanostructured small molecule amphiphile self-assembly materials by design. Chem. Soc. Rev. 41, 1297–1322 (2012).
Sagnella, S. M. et al. Ordered nanostructured amphiphile self-assembly materials from endogenous nonionic unsaturated monoethanolamide lipids in water. Langmuir 26, 3084–3094 (2010).
Negrini, R. & Mezzenga, R. pH-responsive lyotropic liquid crystals for controlled drug delivery. Langmuir 27, 5296–5303 (2011).
Nakano, M. et al. Dispersions of liquid crystalline phases of the monoolein/oleic acid/Pluronic F127 system. Langmuir 18, 9283–9288 (2002).
Engstrom, S., Wadsten-Hindrichsen, P. & Hernius, B. Cubic, sponge, and lamellar phases in the glyceryl monooleyl ether–propylene glycol–water system. Langmuir 23, 10020–10025 (2007).
Phan, S., Fong, W. K., Kirby, N., Hanley, T. & Boyd, B. J. Evaluating the link between self-assembled mesophase structure and drug release. Int. J. Pharmaceut. 421, 176–182 (2011).
Nguyen, T. H., Hanley, T., Porter, C. J. & Boyd, B. J. Nanostructured reverse hexagonal liquid crystals sustain plasma concentrations for a poorly water-soluble drug after oral administration. Drug Deliv. Transl. Res. 1, 429–438 (2011).
Zabara, A., Negrini, R., Baumann, P., Onaca-Fischer, O. & Mezzenga, R. Reconstitution of OmpF membrane protein on bended lipid bilayers: perforated hexagonal mesophases. Chem. Commun. 50, 2642–2645 (2014).
Moore, E. B., de la Llave, E., Welke, K., Scherlis, D. A. & Molinero, V. Freezing, melting and structure of ice in a hydrophilic nanopore. Phys. Chem. Chem. Phys. 12, 4124–4134 (2010).
Osti, N. C. et al. Characteristic features of water dynamics in restricted geometries investigated with quasi-elastic neutron scattering. Chem. Phys. 465, 1–8 (2016).
Molinero, V. & Moore, E. B. Water modeled as an intermediate element between carbon and silicon. J. Phys. Chem. B 113, 4008–4016 (2009).
Moore, E. B. & Molinero, V. Structural transformation in supercooled water controls the crystallization rate of ice. Nature 479, 506–U226 (2011).
Moore, E. B., Allen, J. T. & Molinero, V. Liquid–ice coexistence below the melting temperature for water confined in hydrophilic and hydrophobic nanopores. J. Phys. Chem. C 116, 7507–7514 (2012).
García Fernández, R., Abascal, J. L. & Vega, C. The melting point of ice I h for common water models calculated from direct coexistence of the solid–liquid interface. J. Chem. Phys. 124, 144506 (2006).
Assenza, S. & Mezzenga, R. Curvature and bottlenecks control molecular transport in inverse bicontinuous cubic phases. J. Chem. Phys. 148, 054902 (2018).
Lee, W. B., Mezzenga, R. & Fredrickson, G. H. Anomalous phase sequences in lyotropic liquid crystals. Phys. Rev. Lett. 99, 187801 (2007).
Wu, D. H., Chen, A. D. & Johnson, C. S. An improved diffusion-ordered spectroscopy experiment incorporating bipolar-gradient pulses. J. Magn. Reson. Ser. A 115, 260–264 (1995).
Henchman, R. H. & Cockram, S. J. Water’s non-tetrahedral side. Faraday Discuss. 167, 529–550 (2013).
Acknowledgements
We acknowledge J. S. Siegel for insights and useful discussions, and W. K. Fong for assistance with the SAXS experiments. This work is based on experiments performed at the Swiss spallation neutron source SINQ, Paul Scherrer Institute, Villigen, Switzerland. Support by SNF Sinergia grant CRSII2_154451 to E.M.L. and R.M. is acknowledged. R.M. further acknowledges support from SNF grants 200020_178997 and 200021_172767.
Author information
Authors and Affiliations
Contributions
E.M.L. and R.M. designed and directed the study. L.S.M. developed the lipid synthesis, produced lipidic mesophases, performed SAXS and WAXS experiments, and coordinated the NMR, DSC and FWS experiments. S.A. set up and performed molecular dynamics simulations. M.D. synthesized, purified and performed NMR characterization of the lipids. J.J.V. performed DSC experiments. F.J. collected and interpreted FWS data. S.J. collected and interpreted diffusion NMR data. O.Z. directed the NMR studies. L.S.M., S.A., E.M.L. and R.M. wrote the manuscript with contributions from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information Nature Nanotechnology thanks Tianshu Li and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary text and Supplementary Figures 1–11
Rights and permissions
About this article
Cite this article
Salvati Manni, L., Assenza, S., Duss, M. et al. Soft biomimetic nanoconfinement promotes amorphous water over ice. Nat. Nanotechnol. 14, 609–615 (2019). https://doi.org/10.1038/s41565-019-0415-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-019-0415-0
This article is cited by
-
Dehydration of a crystal hydrate at subglacial temperatures
Nature (2023)
-
Designing cryo-enzymatic reactions in subzero liquid water by lipidic mesophase nanoconfinement
Nature Nanotechnology (2021)
-
Freezing water at constant volume and under confinement
Communications Physics (2020)