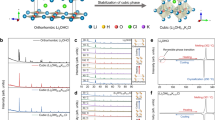
Abstract
Limitations in electrochemical performance as well as supply chain challenges have rendered positive electrode materials a critical bottleneck for Li-ion batteries. State-of-the-art Li-ion batteries fall short of accessing theoretical capacities. As such, there is intense interest in the design of strategies that enable the more effective utilization of active intercalation materials. Pre-intercalation with alkali-metal ions has attracted interest as a means of accessing higher reversible capacity and improved rate performance. However, the structural basis for improvements in electrochemical performance remains mostly unexplored. Here we use topochemical single-crystal-to-single-crystal transformations in a tunnel-structured ζ-V2O5 positive electrode to illustrate the effect of pre-intercalation in modifying the host lattice and altering diffusion pathways. Furthermore, operando synchrotron X-ray diffraction is used to map Li-ion site preferences and occupancies as a function of the depth of discharge in pre-intercalated materials. Na- and K-ion intercalation ‘props open’ the one-dimensional tunnel, reduces electrostatic repulsions between inserted Li ions and entirely modifies diffusion pathways, enabling orders of magnitude higher Li-ion diffusivities and accessing higher capacities. Deciphering the atomistic origins of improved performance in pre-intercalated materials on the basis of single-crystal-to-single-crystal topochemical transformation and operando diffraction studies paves the way to site-selective modification approaches for positive electrode design.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting this study are available within this article and its Supplementary Information. Any additional relevant data are available upon request. Source data are provided with this paper.
References
Cano, Z. P. et al. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 3, 279–289 (2018).
Goodenough, J. B. & Park, K. S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013).
Whittingham, M. S. Lithium batteries: 50 years of advances to address the next 20 years of climate issues. Nano Lett. 20, 8435–8437 (2020).
Whittingham, M. S., Siu, C. & Ding, J. Can multielectron intercalation reactions be the basis of next generation batteries? Acc. Chem. Res. 51, 258–264 (2018).
Ryan, E. M. & Mukherjee, P. P. Mesoscale modeling in electrochemical devices—a critical perspective. Prog. Energy Combust. Sci. 71, 118–142 (2019).
Luo, Y. et al. Effect of crystallite geometries on electrochemical performance of porous intercalation electrodes by multiscale operando investigation. Nat. Mater. 21, 217–227 (2022).
Zhao, Y. et al. A review on modeling of electro-chemo-mechanics in lithium-ion batteries. J. Power Sources 413, 259–283 (2019).
Whittingham, M. S. Lithium batteries and cathode materials. Chem. Rev. 104, 4271–4301 (2004).
Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 11, 1550 (2020).
Liu, L. et al. Alkali ions pre-intercalated layered MnO2 nanosheet for zinc-ions storage. Adv. Energy Mater. 11, 2101287 (2021).
Clites, M. & Pomerantseva, E. Bilayered vanadium oxides by chemical pre-intercalation of alkali and alkali-earth ions as battery electrodes. Energy Storage Mater. 11, 30–37 (2018).
Liu, G. et al. K+ pre-intercalated manganese dioxide with enhanced Zn2+ diffusion for high rate and durable aqueous zinc-ion batteries. J. Mater. Chem. A 7, 20806–20812 (2019).
Chernova, N. A., Roppolo, M., Dillon, A. C. & Whittingham, M. S. Layered vanadium and molybdenum oxides: batteries and electrochromics. J. Mater. Chem. 19, 2526–2552 (2009).
Kumagai, N., Tanno, K., Nakajima, T. & Watanabe, N. Structural changes of Nb2O5 and V2O5 as rechargeable cathodes for lithium battery. Electrochim. Acta 28, 17–22 (1983).
Santos, D. A., Dixit, M. K., Pradeep Kumar, P. & Banerjee, S. Assessing the role of vanadium technologies in decarbonizing hard-to-abate sectors and enabling the energy transition. iScience 24, 103277 (2021).
De Jesus, L. et al. Lithiation across interconnected V2O5 nanoparticle networks. J. Mater. Chem. A 5, 20141–20152 (2017).
Parija, A. et al. Topochemically de-intercalated phases of V2O5 as cathode materials for multivalent intercalation batteries: a first-principles evaluation. Chem. Mater. 28, 5611–5620 (2016).
Horrocks, G. A. et al. Mitigating cation diffusion limitations and intercalation-induced framework transitions in a 1D tunnel-structured polymorph of V2O5. Chem. Mater. 29, 10386–10397 (2017).
De Jesus, L. R., Andrews, J. L., Parija, A. & Banerjee, S. Defining diffusion pathways in intercalation cathode materials: some lessons from V2O5 on directing cation traffic. ACS Energy Lett. 3, 915–931 (2018).
Luo, Y. et al. Roadblocks in cation diffusion pathways: implications of phase boundaries for Li-ion diffusivity in an intercalation cathode material. ACS Appl. Mater. Interfaces 10, 30901–30911 (2018).
De Jesus, L. R. et al. Mapping polaronic states and lithiation gradients in individual V2O5 nanowires. Nat. Commun. 7, 12022 (2016).
Handy, J. V., Luo, Y., Andrews, J. L., Bhuvanesh, N. & Banerjee, S. An atomic view of cation diffusion pathways from single-crystal topochemical transformations. Angew. Chem. Int. Ed. 59, 16385–16392 (2020).
Luo, Y. et al. Cation reordering instead of phase transitions: origins and implications of contrasting lithiation mechanisms in one-dimensional (1D) ζ- and two-dimensional (2D) α-V2O5. Proc. Natl Sci. USA e2115072119 (2022).
Wadsley, A. D. The crystal structure of Na2−xV6O15. Acta Crystallogr. 8, 695–701 (1955).
Clites, M. & Pomerantseva, E. Stabilization of battery electrodes through chemical pre-intercalation of layered materials. Low-Dimens. Mater. Devices 9924, 992405 (2016).
Chen, L. et al. Guest ions pre-intercalation strategy of manganese-oxides for supercapacitor and battery applications. J. Energy Chem. 60, 480–493 (2021).
Mitchell, J. B. et al. Confined interlayer water promotes structural stability for high-rate electrochemical proton intercalation in tungsten oxide hydrates. ACS Energy Lett. 4, 2805–2812 (2019).
Galy, J., Darriet, J., Casalot, A. & Goodenough, J. B. Structure of the MxV2O and MxV2-YTO5 phases. J. Solid State Chem. 1, 339–348 (1970).
Baddour-Hadjean, R., Bach, S., Emery, N. & Pereira-Ramos, J. P. The peculiar structural behaviour of β-Na0.33V2O5 upon electrochemical lithium insertion. J. Mater. Chem. 21, 11296–11305 (2011).
Krogstad, M. J. et al. Reciprocal space imaging of ionic correlations in intercalation compounds. Nat. Mater. 19, 63–68 (2020).
Bach, S., Pereira-Ramos, J. P., Baffier, N. & Messina, R. A thermodynamic and kinetic study of electrochemical lithium intercalation in Na0.33V2O5 bronze prepared by a sol–gel process. J. Electrochem. Soc. 137, 1042–1048 (1990).
Andrews, J. L. et al. Reversible Mg-ion insertion in a metastable one-dimensional polymorph of V2O5. Chem 4, 564–585 (2018).
Li, J. et al. Phase evolution of conversion-type electrode for lithium ion batteries. Nat. Commun. 10, 2224 (2019).
Varghese, B. et al. Fabrication of NiO nanowall electrodes for high performance lithium ion battery. Chem. Mater. 20, 3360–3367 (2008).
He, C. et al. Carbon-encapsulated Fe3O4 nanoparticles as a high-rate lithium ion battery anode material. ACS Nano 7, 4459–4469 (2013).
Wang, G. et al. Mesoporous LiFePO4/C nanocomposite cathode materials for high power lithium ion batteries with superior performance. Adv. Mater. 22, 4944–4948 (2010).
Luo, J. Y., Wang, Y. G., Xiong, H. M. & Xia, Y. Y. Ordered mesoporous spinel LiMn2O4 by a soft-chemical process as a cathode material for lithium-ion batteries. Chem. Mater. 19, 4791–4795 (2007).
Hu, J. et al. 3D-printed cathodes of LiMn1−xFexPO4 nanocrystals achieve both ultrahigh rate and high capacity for advanced lithium-ion battery. Adv. Energy Mater. 6, 1600856 (2016).
Pereira-Ramos, J. P., Messina, R., Znaidi, L. & Baffier, N. Electrochemical lithium intercalation in Na0.33V2O5 bronze prepared by sol–gel processes. Solid State Ion. 28–30, 886–894 (1988).
Handy, J. V. et al. A ‘Li-eye’ view of diffusion pathways in a 2D intercalation material from topochemical single-crystal transformation. ACS Energy Lett. 7, 1960–1962 (2022).
Handy, J. V. et al. Topochemical stabilization and single-crystal transformations of a metastable 2D γʹ-V2O5 intercalation cathode. Cell Rep. Phys. Sci. 3, 100712 (2022).
Yamada, H. & Ueda, Y. Magnetic electric and structural properties of β-AxV2O5 (A = Na, Ag). J. Phys. Soc. Jpn. 68, 2735–2740 (1999).
Chen, B. et al. Electronic structure of β-NaxV2O5(x ≈ 0.33) polycrystalline films: growth spectroscopy and theory. J. Phys. Chem. C 118, 1081–1094 (2014).
Xu, Y., Han, X., Zheng, L., Yan, W. & Xie, Y. Pillar effect on cyclability enhancement for aqueous lithium ion batteries: a new material of β-vanadium bronze M0.33V2O5 (M = Ag, Na) nanowires. J. Mater. Chem. 21, 14466–14472 (2011).
Rajendra, T. et al. Quantifying transport, geometrical, and morphological parameters in Li-ion cathode phases using X-ray microtomography. ACS Appl. Mater. Interfaces 11, 19933–19942 (2019).
Andrews, J. L. et al. Curvature-induced modification of mechano-electrochemical coupling and nucleation kinetics in a cathode material. Matter 3, 1754–1773 (2020).
Schulze, M. C., Belson, R. M., Kraynak, L. A. & Prieto, A. L. Electrodeposition of Sb/CNT composite films as anodes for Li- and Na-ion batteries. Energy Storage Mater. 25, 572–584 (2020).
Parize, J. L., Medouar, A., Savariault, J. M., Ballivet-Tkatchenko, D. & Galy, J. Formation of sodium and copper vanadium oxibronzes via oxalate decomposition: thermal and X ray studies. Mater. Res. Bull. 24, 1147–1153 (1989).
Wizansky, A. R., Rauch, P. E. & Disalvo, F. J. Powerful oxidizing agents for the oxidative deintercalation of lithium from transition-metal oxides. J. Solid State Chem. 81, 203–207 (1989).
Sheldrick, G. M. & IUCr, A. Short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Hübschle, C. B., Sheldrick, G. M. & Dittrich, B. ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 44, 1281–1284 (2011).
Wang, W., Wang, H., Liu, S. & Huang, J. Synthesis of γ-LiV2O5 nanorods as a high-performance cathode for Li ion battery. J. Solid State Electrochem. 16, 2555–2561 (2012).
Deng, Z. et al. 3D ordered macroporous MoS2@C nanostructure for flexible Li-ion batteries. Adv. Mater. 29, 1603020 (2017).
Lee, Y.-S. & Ryu, K.-S. Study of the lithium diffusion properties and high rate performance of TiNb6O17 as an anode in lithium secondary battery. Sci. Rep. 7, 16617 (2017).
Weppner, W. & Huggins, R. A. Determination of the kinetic parameters of mixed-conducting electrodes and application to the system Li3Sb. J. Electrochem. Soc. 124, 1569–1578 (1977).
Piao, T., Park, S., Doh, C. & Moon, S. Intercalation of lithium ions into graphite electrodes studied by AC impedance measurements. J. Electrochem. Soc. 146, 2794 (1999).
Wang, L. et al. Electrochemical impedance spectroscopy (EIS) study of LiNi1/3Co1/3Mn1/3O2 for Li-ion batteries. Int. J. Electrochem. Sci. 7, 345–353 (2012).
Borkiewicz, O. J. et al. The AMPIX electrochemical cell: a versatile apparatus for in situ X-ray scattering and spectroscopic measurements. J. Appl. Crystallogr. 45, 1261–1269 (2012).
Toby, B. H. & Von Dreele, R. B. GSAS-II: the genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 46, 544–549 (2013).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Phys. Rev. 136, B864–B871 (1964).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133 (1965).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Acknowledgements
This study is based on work supported by the National Science Foundation (NSF) under DMR 1809866 (S.B.). This research used resources of the Advanced Photon Source by Argonne National Laboratory under contract number DE-AC02-06CH11357. Argonne National Laboratory is operated for the US Department of Energy Office of Science by UChicago Argonne. We thank K. Wiaderek and A. Yakovenko for their support at beamline 17-BM of the Advanced Photon Source. Use of the TAMU Materials Characterization Facility and the Texas A&M Microscopy and Imaging Center is acknowledged. M.P. acknowledges the support of National Science Foundation (NSF) under DMR 1944674. T.D. and S.C. acknowledge HRI Allahabad and DST-SERB (SRG/2020/001707) for the infrastructure and funding. Computational work for this study was carried out at the cluster computing facility at Harish-Chandra Research Institute (http://www.hri.res.in/cluster).
Author information
Authors and Affiliations
Contributions
Y.L. conceptualized the project under the supervision of S.B. The powder materials were designed, synthesized and characterized and electrochemically tested by Y.L. The operando experiment was conducted by Y.L. with the help of J.V.H. The single-crystal materials were designed, synthesized and characterized by J.V.H., J.D.P. and R.A. The DFT simulations were performed by T.D. under the supervision of S.C. Galvanostatic intermittent titration technique data were acquired by Y.-H.C. Long-term electrochemical cycling was conducted by B.J.S., L.G and D.C.B. Coin cell assembly resources and advice were provided by M.P. The paper was written by Y.L. and revised by S.B., J.V.H. and J.D.P. with the help of all other authors. All authors contributed to discussions and writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
A provisional patent has been filed related to new compositions of lithiated β-Na0.25V2O5/β-K0.22V2O5 by the Texas A&M University System.
Peer review
Peer review information
Nature Materials thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13 and Tables 1–20.
Supplementary Video 1
Single-crystal structure of lithiated sodium vanadium oxide.
Source data
Source Data Fig. 2
Statistical source.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, Y., Handy, J.V., Das, T. et al. Effect of pre-intercalation on Li-ion diffusion mapped by topochemical single-crystal transformation and operando investigation. Nat. Mater. (2024). https://doi.org/10.1038/s41563-024-01842-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41563-024-01842-y