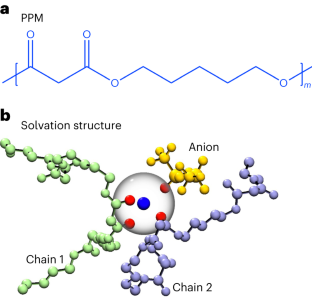
Abstract
Solvation dynamics critically affect charge transport. Spectroscopic experiments and computer simulations show that these dynamics in aqueous systems occur on a picosecond timescale. In the case of organic electrolytes, however, conflicting values ranging from 1 to several 100 picoseconds have been reported. We resolve this conflict by studying mixtures of an organic polymer and a lithium salt. Lithium ions coordinate with multiple polymer chains, resulting in temporary crosslinks. Relaxation of these crosslinks, detected by quasielastic neutron scattering, are directly related to solvation dynamics. Simulations reveal a broad spectrum of relaxation times. The average timescale for solvation dynamics in both experiment and simulation is one nanosecond. We present the direct measurement of ultraslow dynamics of solvation shell break-up in an electrolyte.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The Supplementary Information contains details of QENS and simulation. Further data are available from the corresponding authors upon request. Source data are presented in this paper.
References
Wolynes, P. G. Dynamics of electrolyte solutions. Annu. Rev. Phys. Chem. 31, 345–376 (1980).
Ratner, M. A., Johansson, P. & Shriver, D. F. Polymer electrolytes: ionic transport mechanisms and relaxation coupling. MRS Bull. 25, 31–37 (2000).
Marcus, R. A. On the theory of oxidation‐reduction reactions involving electron transfer. I. J. Chem. Phys. 24, 966–978 (1956).
Bakker, H. Structural dynamics of aqueous salt solutions. Chem. Rev. 108, 1456–1473 (2008).
Bagchi, B. & Jana, B. Solvation dynamics in dipolar liquids. Chem. Soc. Rev. 39, 1936–1954 (2010).
Ohtaki, H. & Radnai, T. Structure and dynamics of hydrated ions. Chem. Rev. 93, 1157–1204 (1993).
He, F. & Richert, R. Solvation dynamics in viscous polymer solution: propylene carbonate confined by poly(methylmethacrylate). Phys. Rev. B 74, 014201 (2006).
Zhang, X.-X., Liang, M., Ernsting, N. P. & Maroncelli, M. Complete solvation response of coumarin 153 in ionic liquids. J. Phys. Chem. B 117, 4291–4304 (2013).
Ben-Amotz, D. Hydration-shell vibrational spectroscopy. J. Am. Chem. Soc. 141, 10569–10580 (2019).
Sacco, A. Structure and dynamics of electrolyte solutions. A NMR relaxation approach. Chem. Soc. Rev. 23, 129–136 (1994).
Pasquarello, A. et al. First solvation shell of the Cu(II) aqua ion: evidence for fivefold coordination. Science 291, 856–859 (2001).
Markovich, G., Perera, L., Berkowitz, M. L. & Cheshnovsky, O. The solvation of Cl−, Br−, and I− in acetonitrile clusters: photoelectron spectroscopy and molecular dynamics simulations. J. Chem. Phys. 105, 2675–2685 (1996).
Kuzmin, A., Obst, S. & Purans, J. X-ray absorption spectroscopy and molecular dynamics studies of hydration in aqueous solutions. J. Phys. Condens. Matter 9, 10065 (1997).
Laage, D. & Hynes, J. T. Reorientional dynamics of water molecules in anionic hydration shells. Proc. Natl Acad. Sci. USA 104, 11167–11172 (2007).
Jimenez, R., Fleming, G. R., Kumar, P. & Maroncelli, M. Femtosecond solvation dynamics of water. Nature 369, 471–473 (1994).
Kropman, M. & Bakker, H. Dynamics of water molecules in aqueous solvation shells. Science 291, 2118–2120 (2001).
Rey, R. & Hynes, J. T. Solvation dynamics in water. 4. On the initial regime of solvation relaxation. J. Phys. Chem. B 124, 7668–7681 (2020).
Lewis, N. H. C., Dereka, B., Zhang, Y., Maginn, E. J. & Tokmakoff, A. From networked to isolated: observing water hydrogen bonds in concentrated electrolytes with two-dimensional infrared spectroscopy. J. Phys. Chem. B 126, 5305–5319 (2022).
Kacenauskaite, L., Van Wyck, S. J., Moncada Cohen, M. & Fayer, M. D. Water-in-salt: fast dynamics, structure, thermodynamics, and bulk properties. J. Phys. Chem. B 128, 291–302 (2024).
Lee, K.-K. et al. Ultrafast fluxional exchange dynamics in electrolyte solvation sheath of lithium ion battery. Nat. Commun. 8, 14658 (2017).
Liang, C., Kwak, K. & Cho, M. Revealing the solvation structure and dynamics of carbonate electrolytes in lithium-ion batteries by two-dimensional infrared spectrum modeling. J. Phys. Chem. Lett. 8, 5779–5784 (2017).
Fulfer, K. D. & Kuroda, D. G. Solvation structure and dynamics of the lithium ion in organic carbonate-based electrolytes: a time-dependent infrared spectroscopy study. J. Phys. Chem. C 120, 24011–24022 (2016).
Fulfer, K. D. & Kuroda, D. G. Ion speciation of lithium hexafluorophosphate in dimethyl carbonate solutions: an infrared spectroscopy study. Phys. Chem. Chem. Phys. 20, 22710–22718 (2018).
Rushing, J. C., Leonik, F. M. & Kuroda, D. G. Effect of solvation shell structure and composition on ion pair formation: the case study of LiTDI in organic carbonates. J. Phys. Chem. C 123, 25102–25112 (2019).
Chen, X., Fulfer, K. D., Woodard, K. T. & Kuroda, D. G. Structure and dynamics of the lithium-ion solvation shell in ureas. J. Phys. Chem. B 123, 9889–9898 (2019).
Lim, J. et al. Two-dimensional infrared spectroscopy and molecular dynamics simulation studies of nonaqueous lithium ion battery electrolytes. J. Phys. Chem. B 123, 6651–6663 (2019).
Galle Kankanamge, S. R. & Kuroda, D. G. Molecular structure, chemical exchange, and conductivity mechanism of high concentration LiTFSI electrolytes. J. Phys. Chem. B 124, 1965–1977 (2020).
Fulfer, K. D., Galle Kankanamge, S. R., Chen, X., Woodard, K. T. & Kuroda, D. G. Elucidating the mechanism behind the infrared spectral features and dynamics observed in the carbonyl stretch region of organic carbonates interacting with lithium ions. J. Chem. Phys. 154, 234504 (2021).
Dereka, B. et al. Exchange-mediated transport in battery electrolytes: ultrafast or ultraslow? J. Am. Chem. Soc. 144, 8591–8604 (2022).
Ganesh, P., Jiang, D.-E. & Kent, P. Accurate static and dynamic properties of liquid electrolytes for Li-ion batteries from ab initio molecular dynamics. J. Phys. Chem. B 115, 3085–3090 (2011).
Zhang, X. & Kuroda, D. G. An ab initio molecular dynamics study of the solvation structure and ultrafast dynamics of lithium salts in organic carbonates: a comparison between linear and cyclic carbonates. J. Chem. Phys. 150, 184501 (2019).
Siegel, D. J., Nazar, L., Chiang, Y.-M., Fang, C. & Balsara, N. P. Establishing a unified framework for ion solvation and transport in liquid and solid electrolytes. Trends Chem. 3, 807–818 (2021).
Yu, X. et al. A practical polymer electrolyte for lithium and sodium batteries: poly(pentyl malonate). ACS Energy Lett. 7, 3791–3797 (2022).
Leibler, L., Rubinstein, M. & Colby, R. H. Dynamics of reversible networks. Macromolecules 24, 4701–4707 (1991).
Rubinstein, M. & Semenov, A. N. Thermoreversible gelation in solutions of associating polymers. 2. Linear dynamics. Macromolecules 31, 1386–1397 (1998).
Kumar, S. K. & Douglas, J. F. Gelation in physically associating polymer solutions. Phys. Rev. Lett. 87, 188301 (2001).
Suzuki, S., Uneyama, T., Inoue, T. & Watanabe, H. Nonlinear rheology of telechelic associative polymer networks: shear thickening and thinning behavior of hydrophobically modified ethoxylated urethane (HEUR) in aqueous solution. Macromolecules 45, 888–898 (2012).
Rapp, P. B., Omar, A. K., Silverman, B. R., Wang, Z.-G. & Tirrell, D. A. Mechanisms of diffusion in associative polymer networks: evidence for chain hopping. J. Am. Chem. Soc. 140, 14185–14194 (2018).
Sinha, K. & Maranas, J. K. Segmental dynamics and ion association in PEO-based single ion conductors. Macromolecules 44, 5381–5391 (2011).
Mongcopa, K. I. S. et al. Relationship between segmental dynamics measured by quasi-elastic neutron scattering and conductivity in polymer electrolytes. ACS Macro Lett. 7, 504–508 (2018).
Rouse, P. E. Jr A theory of the linear viscoelastic properties of dilute solutions of coiling polymers. J. Chem. Phys. 21, 1272–1280 (1953).
Doi, M. & Edwards S.F. The Theory of Polymer Dynamics (Clarendon Press, 1986).
Choo, Y., Halat, D. M., Villaluenga, I., Timachova, K. & Balsara, N. P. Diffusion and migration in polymer electrolytes. Prog. Polym. Sci. 103, 101220 (2020).
Mamontov, E. & Herwig, K. W. A time-of-flight backscattering spectrometer at the Spallation Neutron Source, BASIS. Rev. Sci. Instrum. 82, 085109 (2011).
Arnold, O. et al. Mantid—data analysis and visualization package for neutron scattering and μ SR experiments. Nucl. Instrum. Methods Phys. Res. A 764, 156–166 (2014).
Rubinstein, M. & Semenov, A. N. Dynamics of entangled solutions of associating polymers. Macromolecules 34, 1058–1068 (2001).
Börner, H. et al. Neutron Data Booklet (Institut Laue-Langevin, 2003).
Speight, J. Lange’s Handbook of Chemistry (McGraw-Hill Education, 2005).
Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4418 (2004).
Fang, C., Yu, X., Chakraborty, S., Balsara, N. P. & Wang, R. Molecular origin of high cation transference in mixtures of poly(pentyl malonate) and lithium salt. ACS Macro Lett. 12, 612–618 (2023).
Acknowledgements
This work was supported by the Joint Center for Energy Storage Research (JCESR), an Energy Innovation Hub funded by the US Department of Energy, Office of Science, Office of Basic Energy Science, under contract no. DE-AC02-06CH11357. This research used resources at the Spallation Neutron Source, a US Department of Energy, Office of Science User Facility operated by Oak Ridge National Laboratory. The computations were performed at the Lawrencium cluster at Lawrence Berkeley National Laboratory.
Author information
Authors and Affiliations
Contributions
This project was conceived by N.P.B. and R.W.; N.J.S. designed and conducted the major experiments. C.F. performed the simulation study and all theoretical analysis. N.J.S., N.C.O. and E.M. performed the QENS measurements. X.Y. synthesized the PPM polymer. J.L. prepared the PPM/LiTFSI samples. H.W. guided the analysis of experimental data. The manuscript was written by C.F., N.J.S., R.W. and N.P.B. All authors reviewed the manuscript and approved the final version. The two first authors, N.J.S. and C.F., contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Bogdan Dereka and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, Table 1 and Discussion.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, N.J., Fang, C., Osti, N.C. et al. Nanosecond solvation dynamics in a polymer electrolyte for lithium batteries. Nat. Mater. (2024). https://doi.org/10.1038/s41563-024-01834-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41563-024-01834-y



