Abstract
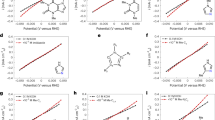
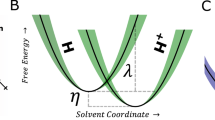
When an electrode contacts an electrolyte, an interfacial electric field forms. This interfacial field can polarize the electrode’s surface and nearby molecules, but its effect can be countered by an applied potential. Quantifying the value of this countering potential (‘potential of zero charge’ (pzc)) is, however, not straightforward. Here we present an optical method for determining the pzc at an electrochemical interface. Our approach uses phase-sensitive second-harmonic generation to determine the electrochemical potential where the interfacial electric field vanishes at an electrode–electrolyte interface with Pt–water as a model experiment. Our method reveals that the pzc of the Pt–water interface is 0.23 ± 0.08 V versus standard hydrogen electrode (SHE) and is pH independent from pH 1 to pH 13. First-principles calculations with a hybrid explicit–implicit solvent model predict the pzc of the Pt(111)–water interface to be 0.23 V versus SHE and reveal how the interfacial water structure rearranges as the electrode potential is moved above and below the pzc. We further show that pzc is sensitive to surface modification; deposition of Ni on Pt shifts the interfacial pzc in the cathodic direction by ~360 mV. Our work demonstrates a materials-agnostic approach for quantifying the interfacial electrical field and water orientation at an electrochemical interface without requiring probe molecules and confirms the long-held view that the interfacial electric field is more intense during hydrogen electrocatalysis in alkaline than in acid.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data for Figs. 2, 3 and 5 are provided in Source data. Any other data are available from the corresponding authors upon reasonable request.
References
Stamenkovic, V. R., Strmcnik, D., Lopes, P. P. & Markovic, N. M. Energy and fuels from electrochemical interfaces. Nat. Mater. 16, 57–69 (2017).
Ledezma-Yanez, I. et al. Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nat. Energy 2, 17031 (2017).
Xu, A., Govindarajan, N., Kastlunger, G., Vijay, S. & Chan, K. Theories for electrolyte effects in CO2 electroreduction. Acc. Chem. Res. 55, 495–503 (2022).
Strmcnik, D. et al. The role of non-covalent interactions in electrocatalytic fuel-cell reactions on platinum. Nat. Chem. 1, 466–472 (2009).
Kelly, S. R., Kirk, C., Chan, K. & Nørskov, J. K. Electric field effects in oxygen reduction kinetics: rationalizing pH dependence at the Pt(111), Au(111), and Au(100) electrodes. J. Phys. Chem. C 124, 14581–14591 (2020).
Lou, S. et al. Insights into interfacial effect and local lithium-ion transport in polycrystalline cathodes of solid-state batteries. Nat. Commun. 11, 5700 (2020).
Rebollar, L. et al. ‘Beyond adsorption’ descriptors in hydrogen electrocatalysis. ACS Catal. 10, 14747–14762 (2020).
Boettcher, S. W. et al. Potentially confusing: potentials in electrochemistry. ACS Energy Lett. 6, 261–266 (2021).
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications (Wiley and Sons, 2000).
Ojha, K., Arulmozhi, N., Aranzales, D. & Koper, M. T. M. Double layer at the Pt(111)–aqueous electrolyte interface: potential of zero charge and anomalous Gouy–Chapman screening. Angew. Chem. Int. Ed. 59, 711–715 (2020).
Cuesta, A. Measurement of the surface charge density of CO-saturated Pt(111) electrodes as a function of potential: the potential of zero charge of Pt(111). Surf. Sci. 572, 11–22 (2004).
Rizo, R., Sitta, E., Herrero, E., Climent, V. & Feliu, J. M. Towards the understanding of the interfacial pH scale at Pt(111) electrodes. Electrochim. Acta 162, 138–145 (2015).
Weaver, M. J. Potentials of zero charge for platinum(111)−aqueous interfaces: a combined assessment from in-situ and ultrahigh-vacuum measurements. Langmuir 14, 3932–3936 (1998).
Sebastián, P., Martínez-Hincapié, R., Climent, V. & Feliu, J. M. Study of the Pt(111) | electrolyte interface in the region close to neutral pH solutions by the laser induced temperature jump technique. Electrochim. Acta 228, 667–676 (2017).
Sarabia, F. J., Sebastián, P., Climent, V. & Feliu, J. M. New insights into the Pt(hkl)-alkaline solution interphases from the laser induced temperature jump method. J. Electroanal. Chem. 872, 114068 (2020).
Climent, V., Attard, G. A. & Feliu, J. M. Potential of zero charge of platinum stepped surfaces: a combined approach of CO charge displacement and N2O reduction. J. Electroanal. Chem. 532, 67–74 (2002).
Martínez-Hincapié, R., Climent, V. & Feliu, J. M. Peroxodisulfate reduction as a probe to interfacial charge. Electrochem. Commun. 88, 43–46 (2018).
Ryu, J. & Surendranath, Y. Tracking electrical fields at the Pt/H2O interface during hydrogen catalysis. J. Am. Chem. Soc. 141, 15524–15531 (2019).
Shen, Y. R. Surface properties probed by second-harmonic and sum-frequency generation. Nature 337, 519–525 (1989).
Corn, R. M. & Higgins, D. A. Optical second harmonic generation as a probe of surface chemistry. Chem. Rev. 94, 107–125 (1994).
Eisenthal, K. B. Liquid interfaces probed by second-harmonic and sum-frequency spectroscopy. Chem. Rev. 96, 1343–1360 (1996).
Chang, H. et al. Direct measurement of charge reversal on lipid bilayers using heterodyne-detected second harmonic generation spectroscopy. J. Phys. Chem. B 124, 641–649 (2020).
Strmcnik, D., Lopes, P. P., Genorio, B., Stamenkovic, V. R. & Markovic, N. M. Design principles for hydrogen evolution reaction catalyst materials. Nano Energy 29, 29–36 (2016).
Sarabia, F. J., Sebastián-Pascual, P., Koper, M. T. M., Climent, V. & Feliu, J. M. Effect of the interfacial water structure on the hydrogen evolution reaction on Pt(111) modified with different nickel hydroxide coverages in alkaline media. ACS Appl. Mater. Interfaces 11, 613–623 (2019).
Xu, P., Huang, A. & Suntivich, J. Phase-sensitive second-harmonic generation of electrochemical interfaces. J. Phys. Chem. Lett. 11, 8216–8221 (2020).
Campbell, D. J., Lynch, M. L. & Corn, R. M. Second harmonic generation studies of anionic chemisorption at polycrystalline platinum electrodes. Langmuir 6, 1656–1664 (1990).
Fredlein, R. A., Damjanovic, A. & Bockris, J. O. Differential surface tension measurements at thin solid metal electrodes. Surf. Sci. 25, 261–264 (1971).
Trasatti, S. & Lust, E. Modern Aspects of Electrochemistry (Kluwer Academic Publishers, 2005).
Tripkovic, V., Björketun, M. E., Skúlason, E. & Rossmeisl, J. Standard hydrogen electrode and potential of zero charge in density functional calculations. Phys. Rev. B 84, 115452 (2011).
Li, C.-Y. Y. et al. In situ probing electrified interfacial water structures at atomically flat surfaces. Nat. Mater. 18, 697–701 (2019).
Wang, Y.-H. et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 600, 81–85 (2021).
Ogasawara, H. et al. Structure and bonding of water on Pt(111). Phys. Rev. Lett. 89, 276102 (2002).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. A. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Mathew, K., Kolluru, V. S. C., Mula, S., Steinmann, S. N. & Hennig, R. G. Implicit self-consistent electrolyte model in plane-wave density-functional theory. J. Chem. Phys. 151, 234101 (2019).
Jinnouchi, R. & Anderson, A. B. Electronic structure calculations of liquid-solid interfaces: combination of density functional theory and modified Poisson-Boltzmann theory. Phys. Rev. B 77, 245417 (2008).
Sakong, S., Forster-Tonigold, K. & Groß, A. The structure of water at a Pt(111) electrode and the potential of zero charge studied from first principles. J. Chem. Phys. 144, 194701 (2016).
Bramley, G., Nguyen, M.-T., Glezakou, V.-A., Rousseau, R. & Skylaris, C.-K. Reconciling work functions and adsorption enthalpies for implicit solvent models: a Pt (111)/water interface case study. J. Chem. Theory Comput. 16, 2703–2715 (2020).
Le, J., Iannuzzi, M., Cuesta, A. & Cheng, J. Determining potentials of zero charge of metal electrodes versus the standard hydrogen electrode from density-functional-theory-based molecular dynamics. Phys. Rev. Lett. 119, 016801 (2017).
Pajkossy, T. & Kolb, D. M. Double layer capacitance of Pt(111) single crystal electrodes. Electrochim. Acta 46, 3063–3071 (2001).
Rossmeisl, J., Nørskov, J. K., Taylor, C. D., Janik, M. J. & Neurock, M. Calculated phase diagrams for the electrochemical oxidation and reduction of water over Pt(111). J. Phys. Chem. B 110, 21833–21839 (2006).
Noguchi, H., Okada, T. & Uosaki, K. SFG study on potential-dependent structure of water at Pt electrode/electrolyte solution interface. Electrochim. Acta 53, 6841–6844 (2008).
Osawa, M., Tsushima, M., Mogami, H., Samjeské, G. & Yamakata, A. Structure of water at the electrified platinum−water interface: a study by surface-enhanced infrared absorption spectroscopy. J. Phys. Chem. C 112, 4248–4256 (2008).
Giles, S. A. et al. Recent advances in understanding the pH dependence of the hydrogen oxidation and evolution reactions. J. Catal. 367, 328–331 (2018).
Paffett, M. T., Campbell, C. T. & Taylor, T. N. Adsorption and growth modes of Bi on Pt(111). J. Chem. Phys. 85, 6176 (1998).
García-Aráez, N., Climent, V. & Feliu, J. M. Evidence of water reorientation on model electrocatalytic surfaces from nanosecond-laser-pulsed experiments. J. Am. Chem. Soc. 130, 3824–3833 (2008).
Haynes, W. M. CRC Handbook of Chemistry and Physics (CRC Press, 2014).
Gileadi, E., Argade, S. D. & Bockris, J. O. The potential of zero charge of platinum and its pH dependence. J. Phys. Chem. 70, 2044–2046 (1966).
Bockris, J. O. M., Argade, S. D. & Gileadi, E. The determination of the potential of zero charge on solid metals. Electrochim. Acta 14, 1259–1283 (1969).
Zheng, J., Nash, J., Xu, B. & Yan, Y. Perspective—towards establishing apparent hydrogen binding energy as the descriptor for hydrogen oxidation/evolution reactions. J. Electrochem. Soc. 165, H27 (2018).
Hall, D. B., Underhill, P. & Torkelson, J. M. Spin coating of thin and ultrathin polymer films. Polym. Eng. Sci. 38, 2039–2045 (1998).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Hammer, B., Hansen, L. B. & Nørskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Filhol, J.-S., Neurock, M., Filhol, J. & Neurock, M. Elucidation of the electrochemical activation of water over Pd by first principles. Angew. Chem. Int. Ed. 45, 402–406 (2006).
Gauthier, J. A. et al. Challenges in modeling electrochemical reaction energetics with polarizable continuum models. ACS Catal. 9, 920–931 (2019).
McNaught, A. D. & Wilkinson, A. The IUPAC Compendium of Chemical Terminology (International Union of Pure and Applied Chemistry, 2019).
Trasatti, S. The absolute electrode potential: an explanatory note (recommendations 1986). Pure Appl. Chem. 58, 955–966 (1986).
Acknowledgements
This work was supported as part of the Center for Alkaline Based Energy Solutions, an Energy Frontier Research Center funded by the US Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences under award #DE-SC0019445. J.S. acknowledges the Sloan Research Fellowship. This work made use of the Cornell Center for Materials Research Shared Facilities which are supported through the Materials Research Science and Engineering Centers program from the National Science Foundation (DMR-1719875). The computational work was performed in part using supercomputing resources from the National Energy Research Scientific Computing Center (BES-ERCAP0019973), a DOE Office of Science User Facility located at Lawrence Berkeley National Laboratory and operated under contract DE-AC02-05CH11231.
Author information
Authors and Affiliations
Contributions
P.X. developed the phase-sensitive second-harmonic generation technique and performed the spectroscopic and electrochemical measurements and data processing. A.D.v.R. and R.S. designed and performed the theoretical modelling work. M.M. and J.S. supervised the project. All authors discussed the results and contributed to writing the manuscript. P.X. and A.D.v.R. contributed equally.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Xihan Chen, Jan Rossmeisl and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19, Discussion and Tables 1–6.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, P., von Rueden, A.D., Schimmenti, R. et al. Optical method for quantifying the potential of zero charge at the platinum–water electrochemical interface. Nat. Mater. 22, 503–510 (2023). https://doi.org/10.1038/s41563-023-01474-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-023-01474-8
This article is cited by
-
Complementary probes for the electrochemical interface
Nature Reviews Chemistry (2024)
-
Measuring the potential of zero charge
Nature Materials (2023)