Abstract
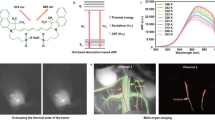
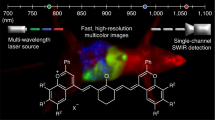
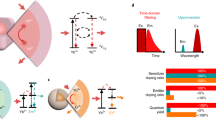
Spectrally distinct fluorophores are desired for multiplexed bioimaging. In particular, monitoring biological processes in living mammals needs fluorophores that operate in the ‘tissue-transparent’ near-infrared (NIR) window, that is, between 700 and 1,700 nm. Here we report a fluorophore system based on molecular erbium(III)–bacteriochlorin complexes with large Stokes shift (>750 nm) and narrowband NIR-to-NIR downconversion spectra (full-width at half-maximum ≤ 32 nm). We have found that the fast (2 × 109 s–¹) and near-unity energy transfer from bacteriochlorin triplets to the erbium(III) 4I13/2 level overcomes the notorious vibrational overtones quenching, resulting in bright and long-lived (1.73 μs) 1,530 nm luminescence in water. We demonstrate the excitation/emission-multiplexed capability of the complexes in the visualization of dynamic circulatory and metabolic processes in living mice, and through skull tracking of cancer cell metastases in mouse brain. This hybrid probe system facilitates robust multiplexed NIR imaging with high contrast and spatial resolution for applications ranging from fluorescence-guided surgery, diagnostics and intravital microscopy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information files and from the corresponding authors upon reasonable request. The crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 1915607 (EB766) and 1915608 (GB762). Source data are provided with this paper.
References
Han, M., Gao, X., Su, J. Z. & Nie, S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat. Biotechnol. 19, 631–635 (2001).
Giepmans, B. N. G., Adams, S. R., Ellisman, M. H. & Tsien, R. Y. The fluorescent toolbox for assessing protein location and function. Science 312, 217–224 (2006).
Grimm, J. B. et al. A general method to fine-tune fluorophores for live-cell and in vivo imaging. Nat. Methods 14, 987–994 (2017).
Zhou, B., Shi, B., Jin, D. & Liu, X. Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol. 10, 924–936 (2015).
Huang, J. et al. Molecular optical imaging probes for early diagnosis of drug-induced acute kidney injury. Nat. Mater. 18, 1133–1143 (2019).
Stack, E. C., Wang, C., Roman, K. A. & Hoyt, C. C. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods 70, 46–58 (2014).
Gao, R. et al. Cortical column and whole-brain imaging with molecular contrast and nanoscale resolution. Science 363, eaau8302 (2019).
Cai, R. et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull–meninges connections. Nat. Neurosci. 22, 317–327 (2019).
Abdeladim, L. et al. Multicolor multiscale brain imaging with chromatic multiphoton serial microscopy. Nat. Commun. 10, 1662 (2019).
Hong, G., Antaris, A. L. & Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1, 0010 (2017).
Antaris, A. L. et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 15, 235–242 (2015).
Hu, Z. et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng. 4, 259–271 (2019).
Wang, S. et al. Anti-quenching NIR-II molecular fluorophores for in vivo high-contrast imaging and pH sensing. Nat. Commun. 10, 1058 (2019).
Cosco, E. D. et al. Flavylium polymethine fluorophores for imaging in the near- and shortwave infrared. Angew. Chem. Int. Ed. 56, 13126–13129 (2017).
Cosco, E. D. et al. Shortwave infrared polymethine fluorophores matched to excitation lasers enable non-invasive, multicolour in vivo imaging in real time. Nat. Chem. 12, 1123–1130 (2020).
Li, Y. et al. Design of AIEgens for near-infrared IIb imaging through structural modulation at molecular and morphological levels. Nat. Commun. 11, 1255 (2020).
Bruns, O. T. et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat. Biomed. Eng. 1, 0056 (2017).
Zhang, M. et al. Bright quantum dots emitting at ∼1,600 nm in the NIR-IIb window for deep tissue fluorescence imaging. Proc. Natl Acad. Sci. USA 115, 6590–6595 (2018).
Eliseeva, S. V. & Bunzli, J.-C. G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 39, 189–227 (2010).
Doffek, C. et al. Understanding the quenching effects of aromatic C–H- and C–D-oscillators in near-IR lanthanoid luminescence. J. Am. Chem. Soc. 134, 16413–16423 (2012).
Ye, H. Q. et al. Organo-erbium systems for optical amplification at telecommunications wavelengths. Nat. Mater. 13, 382–386 (2014).
Mech, A. et al. Sensitized NIR erbium(III) emission in confined geometries: a new strategy for light emitters in telecom applications. J. Am. Chem. Soc. 132, 4574–4576 (2010).
Mancino, G. et al. Dramatic Increases in the lifetime of the Er3+ ion in a molecular complex using a perfluorinated imidodiphosphinate sensitizing ligand. J. Am. Chem. Soc. 127, 524–525 (2005).
Chow, C. Y. et al. Ga3+/Ln3+ metallacrowns: a promising family of highly luminescent lanthanide complexes that covers visible and near-infrared domains. J. Am. Chem. Soc. 138, 5100–5109 (2016).
Trivedi, E. R. et al. Highly emitting near-infrared lanthanide ‘encapsulated sandwich’ metallacrown complexes with excitation shifted toward lower energy. J. Am. Chem. Soc. 136, 1526–1534 (2014).
Nonat, A. et al. Room temperature molecular up conversion in solution. Nat. Commun. 7, 11978 (2016).
Kang, T. S. et al. Near-infrared electroluminescence from lanthanide tetraphenylporphyrin:polystyrene blends. Adv. Mater. 15, 1093–1097 (2003).
Zhang, J., Badger, P. D., Geib, S. J. & Petoud, S. Sensitization of near-infrared-emitting lanthanide cations in solution by tropolonate ligands. Angew. Chem. Int. Ed. 44, 2508–2512 (2005).
Artizzu, F., Mercuri, M. L., Serpe, A. & Deplano, P. NIR-emissive erbium–quinolinolate complexes. Coord. Chem. Rev. 255, 2514–2529 (2011).
Yerushalmi, R., Ashur, I. & Scherz, A. in Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications (eds Grimm, B. et al.) 495–506 (Springer Netherlands, 2006).
Hu, J. Y. et al. Highly near-IR emissive ytterbium(III) complexes with unprecedented quantum yields. Chem. Sci. 8, 2702–2709 (2017).
Garfield, D. J. et al. Enrichment of molecular antenna triplets amplifies upconverting nanoparticle emission. Nat. Photonics 12, 402–407 (2018).
Yang, E. et al. Photophysical properties and electronic structure of stable, tunable synthetic bacteriochlorins: extending the features of native photosynthetic pigments. J. Phys. Chem. B 115, 10801–10816 (2011).
Yao, Y. et al. Aromaticity versus regioisomeric effect of β-substituents in porphyrinoids. Phys. Chem. Chem. Phys. 21, 10152–10162 (2019).
Diao, S. et al. Fluorescence imaging in vivo at wavelengths beyond 1500 nm. Angew. Chem. Int. Ed. 54, 14758–14762 (2015).
Zhong, Y. et al. Boosting the down-shifting luminescence of rare-earth nanocrystals for biological imaging beyond 1500 nm. Nat. Commun. 8, 737 (2017).
Antaris, A. L. et al. A high quantum yield molecule-protein complex fluorophore for near-infrared II imaging. Nat. Commun. 8, 15269 (2017).
Carr, J. A. et al. Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc. Natl Acad. Sci. USA 115, 4465–4470 (2018).
Tian, R. et al. Albumin-chaperoned cyanine dye yields superbright NIR-II fluorophore with enhanced pharmacokinetics. Sci. Adv. 5, eaaw0672 (2019).
Pittet, M. J., Garris, C. S., Arlauckas, S. P. & Weissleder, R. Recording the wild lives of immune cells. Sci. Immunol. 3, eaaq0491 (2018).
Karreman, M. A. et al. Fast and precise targeting of single tumor cells in vivo by multimodal correlative microscopy. J. Cell Sci. 129, 444–456 (2016).
Fan, Y. et al. Lifetime-engineered NIR-II nanoparticles unlock multiplexed in vivo imaging. Nat. Nanotechnol. 13, 941–946 (2018).
Wang, J. C., Murphy, I. A. & Hanson, K. Modulating electron transfer dynamics at dye–semiconductor interfaces via self-assembled bilayers. J. Phys. Chem. C. 119, 3502–3508 (2015).
Starukhin, A., Gorski, A. & Dobkowski, J. Temperature dependence of singlet oxygen generation by different photosensitizers. EPJ Web Conf. 220, 01012 (2019).
Hartzler, D. A. et al. Triplet excited state energies and phosphorescence spectra of (bacterio)chlorophylls. J. Phys. Chem. B 118, 7221–7232 (2014).
Acknowledgements
We thank Xuan Zhang (Techcomp), Mi Wang (Techcomp), Xiaolei Zuo (Shanghai Renji Hospital) and Fangfei Yin (Shanghai Renji Hospital) for their help with absolute quantum yield measurements. F.Z. and D.Z. acknowledge support by the National Key R&D Program of China (grant no. 2017YFA0207303) and the National Natural Science Foundation of China (NSFC, grant nos. 22088101 and 51961145403). F.Z. also acknowledges support by the NSFC (grant no. 21725502) and the Research Program of the Science and Technology Commission of Shanghai Municipality (grant no. 20JC1411700). S.W. acknowledges support by the NSFC (grant no. 22004018) and the China Postdoctoral Science Foundation (grant nos. KLH1615200 and KLH1615215). Y.F. acknowledges support by the NSFC (grant no. 21904023) and the Research Program of the Science and Technology Commission of Shanghai Municipality (grant no. 19490713100). H.Z. acknowledges support by the Research Program of the Science and Technology Commission of Shanghai Municipality (grant no. 20490710600). J.-C.G.B. acknowledges support through a senior visiting scholarship (grant no. 20FGJ04) at the State Key Laboratory of Molecular Engineering of Polymers.
Author information
Authors and Affiliations
Contributions
F.Z., J.-C.G.B. and W.Z. supervised the research. S.W. and F.Z. conceived the research and designed the experiments. Z.L. provided the ligand. T.W. synthesized the complexes and collected data. ZhengXin Wang, ZiYu Wang and W.L. helped with the transient absorption measurements. S.W. and T.W. conducted the optical imaging experiments. H.Z., L.L. and Y.F. built the optical system and helped with image processing. Z.H., P.Y., M.Z. and C.S. contributed to the cell and animal experiments. S.W. and T.W. wrote the manuscript. D.Z. contributed to the cytotoxicity analysis and discussion during the revision process. F.Z. and J.-C.G.B. contributed to the discussion and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Materials thanks Hak Soo Choi, Bruce Cohen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Synthesis of lanthanide-bacteriochlorin complexes.
a, Bacteriochlorin TFPBC was synthesized by a standard solid-state reaction, in which perfluorinated porphyrin (TFPP, 1 g) and an excess of p-toluenesulfonylhydrazide (1:30) were mixed together and heated up to 150 °C for 10 min in absence of oxygen under vacuum. Ln(TFPBC)(LOMe) (Ln = Er: EB766; Ln = Gd: GB762) was prepared by refluxing the precursor Ln[N(SiMe3)2]3 with TFPBC in toluene, followed by in-situ capping with the Kläui’s ligand LOMe. b, The isomer-pure porphyrinoid cis-TFPDL was synthesized following a previously reported method by Zhang et al34. The cis-ErTFPDL was prepared by refluxing the precursor Er(acac)3 with cis-TFPDL in 1,2,4-trichlorobenzene, followed by in-situ capping with the Kläui’s ligand LOMe. EB742 was then obtained from the reduction reaction of cis-ErTFPDL by DIBAL-H in dry THF.
Extended Data Fig. 2 Absorption spectra of EB766 and EB742 in various solvents.
ε denotes the molar extinction coefficient. DCM: dichloromethane, MeOH: methanol, MeCN: acetonitrile. PBS: phosphate buffer, pH = 7.4. EB766 and EB742 cannot dissolve directly in water, but can stably disperse in PBS with the help of DSPE-mPEG2000 phospholipid micelles or bovine serum albumin (BSA).
Extended Data Fig. 3 Investigation of the EB766 phospholipid micelles.
a, Schematic showing the formation of EB766 phospholipid micelles with the surfactants DSPE-mPEG2000. The micelles have an average hydrodynamic diameter of ~15 nm with a hydrophobic cavity of ~5 nm according to the DLS data. b, Photostability of EB766 phospholipid micelles in PBS buffer (pH 7.4, 1×) under continuous 760 nm (2.3 W cm−2) exposure for 30 minutes. Indocyanine green (ICG) was chosen as reference. Solid lines represent mean values, shaded areas represent s.d. (3 replicated measurements). c-e, Size distribution of EB766 phospholipid micelles at day 0 and 7 in (c) PBS buffer (pH 7.4, 1×), (d) 10% FBS and (e) blood serum, respectively, determined by dynamic light scattering (DLS). f, Normalized absorbance of EB766 phospholipid micelles versus time in various media (1× PBS, 10% FBS and blood serum). The bars represent mean ± s.d. derived from n = 3 independent experimental groups. g-h, Absorption spectra (g) and luminescence spectra (h) of EB766 phospholipid micelles in various pH values, showing that EB766 phospholipid micelles has considerable stability in the physiological environment.
Extended Data Fig. 4 Representative luminescence decay curves of EB766 (10 μM) measured at 1530 nm in different solvents.
a, in dichloromethane and deuterated dichloromethane. b, in toluene. c, in methanol and deuterated methanol. d, in acetonitrile and deuterated acetonitrile. e, in PBS and deuterated water. The sample in PBS is a micelle formulation (see Methods, Extended Data Fig. 3). All the lifetime measurements have been replicated for more than three times. The mean values and corresponding standard deviations have been summarized in Supplementary Table 2.
Extended Data Fig. 5 Nanosecond transient absorption spectra for TFPBC and GB762.
a-c, Nanosecond transient absorption (TA) contour maps of TFPBC (a) and GB762 (b) in deaerated dimethylformamide, and GB762 (c) in aerated dimethylformamide, using 345-nm pulsed laser excitation [20 nJ per pulse, 50 fs full width at half maximum (FWHM); ∆A, change in absorbance]. d–f, TA kinetics monitored at 391 nm for TFPBC (d), and 404 nm for GB762 in deaerated (e) and aerated (f) dimethylformamide, respectively, with their respective single exponential fit lines (solid). The results show that the T1 lifetimes for TFPBC in deaerated dimethylformamide, and GB762 in deaerated (τdeaerated) and aerated (τaerated) dimethylformamide are 12.48 μs, 389 and 191 ns, respectively. Oxygen quenching rate{kq[O2]} of T1 can be estimated by the equation: kq[O2] = 1/τaerated − 1/τdeaerated.
Extended Data Fig. 6 Femtosecond transient absorption spectra for TFPBC.
a, Left panel: Femtosecond transient absorption (TA) contour map of TFPBC in deaerated dimethylformamide, using 345-nm pulsed laser excitation [20 nJ per pulse, 50 fs full width at half maximum (FWHM); ∆A, change in absorbance]. Right panel: TA kinetics monitored at 393 (red) and 450 nm (blue), respectively, with their respective multiexponential fit lines (solid). b, Normalized decay-associated difference spectra (DADS) of TFPBC obtained by performing singular value decomposition (SVD) and global fitting of the femtosecond TA raw data, yielding three components (S2, S1 and T1) with their respective lifetimes. The long time constant (blue) was determined independently by nanosecond flash photolysis. Femtosecond TA contour map show fast dynamic processes over the first 10 picoseconds, followed by slow kinetic changes with similar spectral evolution as GB762. In the time range of 10–7.5 ns, the excited state absorption (ESA) centered at 450 nm decayed, coinciding with the growth of the ESA centered at 393 nm. The DADS of TFPBC shows one more short-lived component (0.9 ps) than that of EB766 and GB762. This component can be assigned to the second excited state (S2), directly generated by 345-nm laser excitation. The other two components correspond to the S1 (3.43 ns) and T1 (12.48 μs, obtained by nanosecond flash photolysis) levels, respectively. Because of the heavy atoms effects of Er(III) and Gd(III) ions, the rate constants for S2-to-S1 interconversion in the complexes are too fast to be fitted by global analysis.
Extended Data Fig. 7 Other spectroscopic evidences for the triplet sensitization mechanism.
a, Normalized oxygen-dependent emission spectra of EB766 at 298K in deaerated and aerated dichloromethane (concentration: 10 μM, excitation: 760 nm). The emission intensity of EB766 in the 1450–1600 nm range is oxygen-independent, and no singlet oxygen emission was detected, indicating that the triplet energy transfer rate is much faster than oxygen quenching rate. b, Normalized temperature-dependent emission spectra of EB766 in aerated 2-methyltetrahydrofuran (from 77K to 298K, increment: 20K, concentration: 10 μM, excitation: 760 nm). The inset shows the normalized integral intensity of emission spectra in the 1400–1600 nm range versus temperature. The emission spectra in the 1400–1600 nm range show complicated variations of peak components over temperature, but the corresponding integral intensity show minor change, indicating that there is little energy back transfer in the sensitization process. c, Normalized emission spectra of GB762 at 77K (red) and 298K (blue) in aerated 2-methyltetrahydrofuran. d, Emission decay curves of GB762 measured at 1250 nm (77K, red) and 1270 nm (298K, blue), corresponding to lifetimes of 16.9 and 41.6 μs. The emission around 1270 nm (298K) is the characteristic photoluminescence of singlet oxygen, which has been enhanced by the heavy atom effect of Gd(III) ion. Since singlet oxygen is practically not generated at 77K44, the emission around 1250 nm (77K) should be assigned to the ligand phosphorescence, consistent with some previous reports that the phosphorescence peaks bacteriochlorins locate beyond 1100 nm45.
Extended Data Fig. 8 In vivo NIR-II vascular imaging with EB766.
a, Schematic illustration showing the protocol of NIR-II wide-field whole-body imaging for b and d. Excitation: 760 nm/55 mW cm−2. Emission collection: 1400–1600 nm. Exposure time: 100 ms. b, Cerebral vascular image of nude mouse taken through intact scalp. Scale bar, 2 mm. c, Cross-sectional intensity profile along the red bar in b. d, Hindlimb vascular image of nude mouse. Scale bar, 2 mm. Experiments were repeated for n = 3 biologically independent mice. e, Cross-sectional fluorescence intensity profile (black) and Gaussian fit (red) along the dashed bar in d, clearly showing the closely spaced femoral artery and vein at ∼180 μm distance. f, Schematic illustration showing the protocol of NIR-II intravital microscopic imaging for g–i. Excitation: 760 nm/100 mW. Emission collection: 1400–1600 nm. Exposure time: 200 ms. g, Low-magnification cerebral vascular image of nude mouse taken through intact skull, with a field of view of 38.4 mm × 30.72 mm. Scale bar, 3 mm. h, Zoomed-in image of a sub-region in g taken by a 10× dry objective, with a field of view of 1.28 mm × 1.02 mm. Scale bar, 100 μm. i, Zoomed-in image of a sub-region in h taken by a 25× water-immersion objective, with a field of view of 0.5 mm × 0.36 mm. Scale bar: 50 μm. j, Cross-sectional fluorescence intensity profile (black) and Gaussian fit (red) along the dashed bar in i. Signal-to-noise ratios (SNR) were calculated by dividing the peak intensity values by the defined noise value. Representative images were repeated for n = 3 biologically independent mice.
Extended Data Fig. 9 Investigation of the interactions between EB766 and BSA.
a, Schematic illustration shows that EB766 forms nanoaggregates in aqueous solution, but forms a complex in the presence of BSA. b, Normalized absorption and luminescence spectra of EB766 nanoaggregates and EB766/BSA complexes in PBS buffer (pH 7.4, 1×), showing that both the absorption and luminescence enhanced when EB766 interacted with BSA. c, Size distribution of EB766 nanoaggregates (~250 nm) and EB766/BSA complexes (~7 nm) in PBS buffer (pH 7.4, 1×), determined by dynamic light scattering (DLS). d, Change in fluorescence intensity of BSA (10 μM, excited at 280 nm) in PBS buffer (pH 7.4, 1×) upon titration with EB766 at various BSA/EB766 molar ratio (5.0, 4.0, 3.0, 2.0 and 1.0). The emission intensity of BSA tryptophan residues at 340 nm shows gradual decrease and red-shift upon titration with EB766. e, Corresponding Lineweaver-Burk plot of a. f-h, Photostability of EB766 in (f) PBS buffer (pH 7.4, 1×), in the presence of (g) BSA and (h) 10% FBS under continuous 760 nm (2.3 W cm−2) exposure for 30 minutes. Solid lines represent mean values, shaded areas represent s.d. (3 replicated measurements).
Extended Data Fig. 10 Live cell imaging with CT1530.
a, Schematic illustration showing the protocol of live cell imaging with NIR-II wide-field inverted epifluorescence microscope. (b–f) The fluorescence signals (900–1700 nm) were continuously collected at different time points after adding fresh cell culture using a 40× air-immersion objective, with a field of view of 0.31 mm × 0.19 mm. Scale bar, 20 μm. Experiments were repeated for n = 3 biologically independent groups.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11, Tables 1–7, Methods, notes and references.
Supplementary Video 1
Wide-field whole-body imaging of lymphatic drainage in a living mouse.
Supplementary Video 2
Wide-field whole-body imaging of peristaltic gastrointestinal tract in a living mouse.
Supplementary Video 3
Intravital microscopic imaging of cancer cells movement in mouse brain in vivo.
Supplementary Data 1
Crystallographic data for EB766: CCDC reference 1915607.
Supplementary Data 2
Crystallographic data for GB762: CCDC reference 1915608.
Supplementary Data 3
Statistical source data for Supplementary Figs. 8 and 9.
Source data
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, T., Wang, S., Liu, Z. et al. A hybrid erbium(III)–bacteriochlorin near-infrared probe for multiplexed biomedical imaging. Nat. Mater. 20, 1571–1578 (2021). https://doi.org/10.1038/s41563-021-01063-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-021-01063-7
This article is cited by
-
Near-infrared II fluorescence imaging
Nature Reviews Methods Primers (2024)
-
Ultra-photostable small-molecule dyes facilitate near-infrared biophotonics
Nature Communications (2024)
-
In vivo NIR-II fluorescence imaging for biology and medicine
Nature Photonics (2024)
-
Noninvasive in vivo microscopy of single neutrophils in the mouse brain via NIR-II fluorescent nanomaterials
Nature Protocols (2024)
-
In situ orderly self-assembly strategy affording NIR-II-J-aggregates for in vivo imaging and surgical navigation
Nature Communications (2023)