Abstract
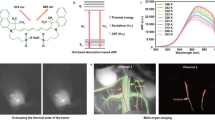
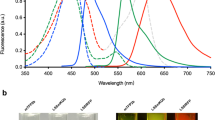
High-resolution, multiplexed experiments are a staple in cellular imaging. Analogous experiments in animals are challenging, however, due to substantial scattering and autofluorescence in tissue at visible (350–700 nm) and near-infrared (700–1,000 nm) wavelengths. Here, we enable real-time, non-invasive multicolour imaging experiments in animals through the design of optical contrast agents for the shortwave infrared (SWIR, 1,000–2,000 nm) region and complementary advances in imaging technologies. We developed tunable, SWIR-emissive flavylium polymethine dyes and established relationships between structure and photophysical properties for this class of bright SWIR contrast agents. In parallel, we designed an imaging system with variable near-infrared/SWIR excitation and single-channel detection, facilitating video-rate multicolour SWIR imaging for optically guided surgery and imaging of awake and moving mice with multiplexed detection. Optimized dyes matched to 980 nm and 1,064 nm lasers, combined with the clinically approved indocyanine green, enabled real-time, three-colour imaging with high temporal and spatial resolutions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Image datasets, including all raw and processed imaging data generated in this work, are available at BioImage Archive (accession number: S-BIAD27). All other datasets that support the findings of this study are contained within the manuscript and its Supplementary Information.
Code availability
Custom computer programs used for the work are available at GitHub (https://gitlab.com/brunslab/ccda).
References
Dean, K. M. & Palmer, A. E. Advances in fluorescence labeling strategies for dynamic cellular imaging. Nat. Chem. Biol. 10, 512–523 (2014).
Wei, L. et al. Super-multiplex vibrational imaging. Nature 544, 465–470 (2017).
Frangioni, J. V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 7, 626–634 (2003).
Kong, L. et al. Continuous volumetric imaging via an optical phase-locked ultrasound lens. Nat. Methods 12, 759–762 (2015).
Grimm, J. B. et al. A general method to fine-tune fluorophores for live-cell and in vivo imaging. Nat. Methods 14, 987–994 (2017).
Fenrich, K. K. et al. Long-term in vivo imaging of normal and pathological mouse spinal cord with subcellular resolution using implanted glass windows. J. Physiol. 590, 3665–3675 (2012).
Rakhymzhan, A. et al. Synergistic strategy for multicolor two-photon microscopy: application to the analysis of germinal center reactions in vivo. Sci. Rep. 7, 7101 (2017).
Kim, P., Puoris’haag, M., Côté, D., Lin, C. P. & Yun, S. H. In vivo confocal and multiphoton microendoscopy. J. Biomed. Opt. 13, 010501 (2008).
Kosaka, N., Ogawa, M., Sato, N., Choyke, P. L. & Kobayashi, H. In vivo real-time, multicolor, quantum dot lymphatic imaging. J. Invest. Dermatol. 129, 2818–2822 (2009).
Erogbogbo, F. et al. In vivo targeted cancer imaging, sentinel lymph node mapping and multi-channel imaging with biocompatible silicon nanocrystals. ACS Nano 5, 413–423 (2011).
Shcherbakova, D. M. & Verkhusha, V. V. Near-infrared fluorescent proteins for multicolor in vivo imaging. Nat. Methods 10, 751–754 (2013).
Zavaleta, C. L. et al. Multiplexed imaging of surface enhanced Raman scattering nanotags in living mice using noninvasive Raman spectroscopy. Proc. Natl Acad. Sci. USA 106, 13511–13516 (2009).
Zhang, H. et al. Penetration depth of photons in biological tissues from hyperspectral imaging in shortwave infrared in transmission and reflection geometries. J. Biomed. Opt. 21, 126006 (2016).
Carr, J. A. et al. Absorption by water increases fluorescence image contrast of biological tissue in the shortwave infrared. Proc. Natl Acad. Sci. USA 115, 9080–9085 (2018).
Naczynski, D. J. et al. Rare-earth-doped biological composites as in vivo shortwave infrared reporters. Nat. Commun. 4, 2199 (2013).
Welsher, K. et al. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat. Nanotechnol. 4, 773–780 (2009).
Hong, G. et al. In vivo fluorescence imaging with Ag2S quantum dots in the second near-infrared region. Angew. Chem. Int. Ed. 51, 9818–9821 (2012).
Bruns, O. T. et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat. Biomed. Eng. 1, 0056 (2017).
Tao, Z. et al. Biological imaging using nanoparticles of small organic molecules with fluorescence emission at wavelengths longer than 1000 nm. Angew. Chem. Int. Ed. 52, 13002–13006 (2013).
Yang, Q. et al. Rational design of molecular fluorophores for biological imaging in the NIR-II window. Adv. Mater. 29, 1605497 (2017).
Zhu, S. et al. 3D NIR-II molecular imaging distinguishes targeted organs with high-performance NIR-II bioconjugates. Adv. Mater. 30, 1705799 (2018).
Wan, H. et al. A bright organic NIR-II nanofluorophore for three-dimensional imaging into biological tissues. Nat. Commun. 9, 1171 (2018).
Wang, F. et al. Light-sheet microscopy in the near-infrared II window. Nat. Methods 16, 545–552 (2019).
Zhu, S. et al. Molecular imaging of biological systems with a clickable dye in the broad 800- to 1,700-nm near-infrared window. Proc. Natl Acad. Sci. USA 114, 962–967 (2017).
Ma, B. L., Zhai, X., Du, G. & Zhou, J. Orthogonal shortwave infrared emission based on rare earth nanoparticles for interference-free logical codes and bio-imaging. Chem. Sci. 10, 3281–3288 (2019).
Hong, G. et al. Through-skull fluorescence imaging of the brain in a new near-infrared window. Nat. Photonics 8, 723–730 (2014).
Diao, S. et al. Fluorescence imaging in vivo at wavelengths beyond 1500 nm. Angew. Chem. Int. Ed. 54, 14758–14762 (2015).
Kapanidis, A. N. et al. Alternating-laser excitation of single molecules. Acc. Chem. Res. 38, 523–533 (2005).
Müller, B. K., Zaychikov, E., Bräuchle, C. & Lamb, D. C. Pulsed interleaved excitation. Biophys. J. 89, 3508–3522 (2005).
Lewis, E. K. et al. Color-blind fluorescence detection for four-color DNA sequencing. Proc. Natl Acad. Sci. USA 102, 5346–5351 (2005).
Garbacik, E. T., Sanz-Paz, M., Borgman, K. J. E., Campelo, F. & Garcia-Parajo, M. F. Frequency-encoded multicolor fluorescence imaging with single-photon-counting color-blind detection. Biophys. J. 115, 725–736 (2018).
Gómez-García, P. A., Garbacik, E. T., Otterstrom, J. J., Garcia-Parajo, M. F. & Lakadamyali, M. Excitation-multiplexed multicolor superresolution imaging with fm-STORM and fm-DNA-PAINT. Proc. Natl Acad. Sci. USA 115, 12991–12996 (2018).
Thimsen, E., Sadtler, B. & Berezin, M. Y. Shortwave-infrared (SWIR) emitters for biological imaging: a review of challenges and opportunities. Nanophotonics 6, 1043–1054 (2017).
Bricks, J. L., Kachkovskii, A. D., Slominskii, Y. L., Gerasov, A. O. & Popov, S. V. Molecular design of near infrared polymethine dyes: a review. Dyes Pigm. 121, 238–255 (2015).
Marshall, M. V. et al. Near-infrared fluorescence imaging in humans with indocyanine green: a review and update. Open Surg. Oncol. J. 2, 12–25 (2010).
Rurack, K. & Spieles, M. Fluorescence quantum yields of a series of red and near-infrared dyes emitting at 600–1000 nm. Anal. Chem. 83, 1232–1242 (2011).
Carr, J. A. et al. Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc. Natl Acad. Sci. USA 115, 4465–4470 (2018).
Starosolski, Z. et al. Indocyanine green fluorescence in second near-infrared (NIR-II) window. PLOS One 12, e0187563 (2017).
Cosco, E. D. et al. Flavylium polymethine fluorophores for near- and shortwave infrared imaging. Angew. Chem. Int. Ed. 56, 13126–13129 (2017).
Henary, M., Mojzych, M., Say, M. & Strekowski, L. Functionalization of benzo[c,d]indole system for the synthesis of visible and near-infrared dyes. J. Heterocycl. Chem. 46, 84–87 (2009).
Li, B., Lu, L., Zhao, M., Lei, Z. & Zhang, F. An efficient 1064 nm NIR-II excitation fluorescent molecular dye for deep-tissue high-resolution dynamic bioimaging. Angew. Chem. Int. Ed. 57, 7483–7487 (2018).
Ding, B. et al. Polymethine thiopyrylium fluorophores with absorption beyond 1000 nm for biological imaging in the second near-infrared subwindow. J. Med. Chem. 62, 2049–2059 (2019).
Wang, S. et al. Anti-quenching NIR-II molecular fluorophores for in vivo high-contrast imaging and pH sensing. Nat. Commun. 10, 1058 (2019).
Lei, Z. et al. Stable, wavelength-tunable fluorescent dyes in the NIR-II region for in vivo high-contrast bioimaging and multiplexed biosensing. Angew. Chem. Int. Ed. 58, 8166–8171 (2019).
Chen, J.-R., Wong, J.-B., Kuo, P.-Y. & Yang, D.-Y. Synthesis and characterization of coumarin-based spiropyran photochromic colorants. Org. Lett. 10, 4823–4826 (2008).
Roehri-Stoeckel, C., Gonzalez, E. & Fougerousse, A. Synthetic dyes: simple and original ways to 4-substituted flavylium salts and their corresponding vitisin derivatives. Can. J. Chem. 79, 1173–1178 (2001).
Seijas, J. A., Vázquez-Tato, M. P. & Carballido-Reboredo, R. Solvent-free synthesis of functionalized flavones under microwave irradiation. J. Org. Chem. 70, 2855–2858 (2005).
Kónya, K., Pajtás, D., Kiss-Szikszai, A. & Patonay, T. Buchwald-Hartwig reactions of monohaloflavones. Eur. J. Org. Chem. 2015, 828–839 (2015).
Kopainsky, B., Qiu, P., Kaiser, W., Sens, B. & Drexhage, K. H. Lifetime, photostability, and chemical structure of IR heptamethine cyanine dyes absorbing beyond 1 μm. Appl. Phys. B 29, 15–18 (1982).
Hansch, C., Leo, A. & Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Semonin, O. E. et al. Absolute photoluminescence quantum yields of IR-26 Dye, PbS, and PbSe quantum dots. J. Phys. Chem. Lett. 1, 2445–2450 (2010).
Hatami, S. et al. Absolute photoluminescence quantum yields of IR26 and IR-emissive Cd1−xHgxTe and PbS quantum dots – method- and material-inherent challenges. Nanoscale 7, 133–143 (2015).
Lukasik, V. M. & Gillies, R. J. Animal anaesthesia for in vivo magnetic resonance. NMR Biomed. 16, 459–467 (2003).
Schwenke, D. O., Pearson, J. T., Mori, H. & Shirai, M. Long-term monitoring of pulmonary arterial pressure in conscious, unrestrained mice. J. Pharmacol. Toxicol. Methods 53, 277–283 (2006).
Sato, M. et al. Simultaneous monitoring of mouse respiratory and cardiac rates through a single precordial electrode. J. Pharmacol. Sci. 137, 177–186 (2018).
Ceppi, L. et al. Real-time single-walled carbon nanotube-based fluorescence imaging improves survival after debulking surgery in an ovarian cancer model. ACS Nano 13, 5356–5365 (2019).
Zhu, S., Tian, R., Antaris, A. L., Chen, X. & Dai, H. Near‐infrared‐II molecular dyes for cancer imaging and surgery. Adv. Mater. 31, e1900321 (2019).
Cousins, A., Thompson, S. K., Wedding, A. B. & Thierry, B. Clinical relevance of novel imaging technologies for sentinel lymph node identification and staging. Biotechnol. Adv. 32, 269–279 (2014).
Rasmussen, J. C., Fife, C. E. & Sevick-Muraca, E. M. Near-infrared fluorescence lymphatic imaging in lymphangiomatosis. Lymphat. Res. Biol. 13, 195–201 (2015).
Tozzi, M., Boni, L., Soldini, G., Franchin, M. & Piffaretti, G. Vascular fluorescence imaging control for complex renal artery aneurysm repair using laparoscopic nephrectomy and autotransplantation. Case Rep. Transplant. 2014, 563408 (2014).
Bexrud, J. A., Eisenberger, P., Leitch, D. C., Payne, P. R. & Schafer, L. L. Selective C-H activation α to primary amines. Bridging metallaaziridines for catalytic, intramolecular α-alkylation. J. Am. Chem. Soc. 131, 2116–2118 (2009).
Kövér, J. & Antus, S. Facile deoxygenation of hydroxylated flavonoids by palladium-catalysed reduction of its triflate derivatives. Z. Naturforsch. 60b, 792–796 (2005).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Rueden, C. T. et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinf. 18, 529 (2017).
Acknowledgements
This work was supported by grants to E.D.C. (NSF GRFP DGE-1144087, Christopher S. Foote Fellowship), O.T.B. (Emmy-Noether-Programme of DFG BR 5355/2-1, Helmholtz Pioneer Campus Institute for Biomedical Engineering), E.M.S. (UCLA, Sloan Research Award FG-2018-10855, NIH 1R01EB027172-01) and Helmholtz Zentrum München, and by shared instrumentation grants from the NSF (CHE-1048804) and NIH (1S10OD016387-01). We thank T. Schwarz-Romond, T. S. Bischof, D. Bengel (Helmholtz Zentrum München), K. N. Houk, J. R. Caram (UCLA) and L. D. Lavis (Janelia) for discussions and support and the Garcia-Garibay Group (UCLA) for instrumentation.
Author information
Authors and Affiliations
Contributions
E.D.C., E.M.S. and O.T.B. designed the study. E.M.S. and O.T.B. jointly advised the study. E.D.C., A.L.S., M.P. and R.R.M. performed the synthesis. E.D.C. measured and analysed the photophysics. S.R. developed the software and built electronics and instrumentation. E.D.C., J.G.P.L. and M.W. built optical configurations. E.D.C., M.S., B.A.A. and S.G. performed imaging experiments. E.D.C., J.G.P.L. and B.A.A. analysed images. K.C.Y.W. performed cell culture experiments. E.D.C., E.M.S. and O.T.B. wrote and edited the paper. E.M.S., O.T.B. and V.N. provided funding. All authors have given approval to the final version of the manuscript. Correspondence and requests for materials should be addressed to E.M.S and O.T.B.
Corresponding authors
Ethics declarations
Competing interests
Material presented in this work is included in patent and patent applications US20200140404, EP3634397 and WO2018226720 with authors E.M.S and E.D.C. and PCT/EP2020065753 with authors O.T.B., E.D.C., J.G.P.L., M.W., S.R., M.S. and E.M.S.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Retrosynthesis of 7-aminoflavylium heterocycles.
The 7-amino-4-methyl flavylium heterocycles were accessed through flavone intermediates 13. Three routes were used to obtain flavones 13, Mentzer pyrone synthesis, Buchwald-Hartwig coupling, and acylation. Conditions and yields for each derivative can be found in the listed Supplementary Tables.
Extended Data Fig. 2 Resolution effects upon excitation multiplexing and single-channel detection in the SWIR.
a, Images of a mouse phantom acquired after injection of JuloFlav7 (3) (94 nmol) and immediate euthanasia. Images were acquired with 785 nm excitation (93 mWcm−2), 980 nm (30 mWcm−2), and 1,064 nm (11 mWcm−2) and collection 1,150–1,700 nm (48 ms, 20 fps). Displayed images are averaged over 200 frames. b, To observe resolution, 12 cross-sections were drawn over different vessels (labelled in a) and the baseline subtracted and normalized cross-sections are overlaid. ex. = excitation; LP = longpass.
Extended Data Fig. 3 Resolution effects observed upon emission multiplexing with a single excitation wavelength in the SWIR.
a, Images of a mouse phantom acquired after injection of JuloFlav7 (3) (94 nmol) and immediate euthanasia, after one freeze-thaw cycle. Images were acquired with 980 nm excitation. Laser powers and exposure time are as follows (1) 1,000 nm LP = 8.1 mWcm−2, 30 ms; 1,300 nm LP = 24 mWcm−2, 500 ms; 1,500 nm LP = 177 mWcm−2, 500 ms. Displayed images are averaged over 200 frames. b, To observe resolution, 5 cross-sections were drawn over different vessels (labelled in a) and the baseline subtracted and normalized cross-sections are overlaid. LP = longpass.
Extended Data Fig. 4 Imaging with 1064 nm excitation after injection of JuloFlav7 (3) micelles.
a, Whole mouse imaging at selected time-points after i.v. injection of JuloFlav7 (3) micelles (44 nmol) in PBS buffer with excitation at 1,064 nm (103 mWcm−1) and 1,150–1,700 nm collection (8 ms exposure time, 100 fps). b, Imaging of the mouse hind-limb at selected time points after i.v. injection of JuloFlav7 (3) micelles (55 nmol) with excitation at 1,064 nm (95 mWcm−1) with 1,100–17,00 nm collection (9 ms exposure time, 100 fps). Displayed images are averaged over 5 frames. Scale bars represent 1 cm. Data are representative of two replicate experiments.
Supplementary information
Supplementary Information
Abbreviations, Supplementary Tables 1–5, Figs. 1–16, List of Supplementary Videos 1–6, figure experimental procedures, extended data and supplementary figure experimental procedures, Notes 1–5, synthetic procedures and compound characterization.
Supplementary Video 1
Single-channel in vivo imaging with excitation 1,064 nm at 100 fps. Image sequence was frame averaged by a factor of three to reduce file size and is displayed in real time.
Supplementary Video 2
Video-rate multiplexed imaging in vivo of JuloFlav7 injection. Image sequence was frame averaged by a factor of three to reduce file size and is displayed at three times the speed.
Supplementary Video 3
Video-rate multiplexed imaging in vivo of ICG injection. Image sequence was frame averaged by a factor of three to reduce file size and is displayed at three times the speed.
Supplementary Video 4
Imaging of an awake mouse in three colours. Image sequence was not frame averaged and is displayed in real time.
Supplementary Video 5
Orthogonal circulatory and lymphatic imaging. Image sequence was frame averaged by a factor of two to reduce file size and is displayed at three times the speed.
Supplementary Video 6
Two-colour demonstration of image-guided necropsy. Image sequence was frame averaged by a factor of eight to reduce file size and is displayed at five times the speed.
Rights and permissions
About this article
Cite this article
Cosco, E.D., Spearman, A.L., Ramakrishnan, S. et al. Shortwave infrared polymethine fluorophores matched to excitation lasers enable non-invasive, multicolour in vivo imaging in real time. Nat. Chem. 12, 1123–1130 (2020). https://doi.org/10.1038/s41557-020-00554-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00554-5
This article is cited by
-
Silver telluride colloidal quantum dot infrared photodetectors and image sensors
Nature Photonics (2024)
-
Biomimetic NIR-II fluorescent proteins created from chemogenic protein-seeking dyes for multicolor deep-tissue bioimaging
Nature Communications (2024)
-
Near-infrared II fluorescence imaging
Nature Reviews Methods Primers (2024)
-
A general strategy to develop fluorogenic polymethine dyes for bioimaging
Nature Chemistry (2024)
-
In vivo NIR-II fluorescence imaging for biology and medicine
Nature Photonics (2024)