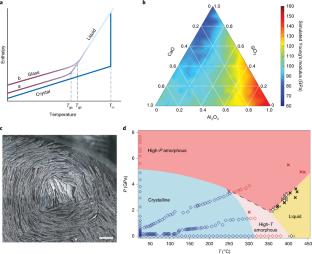
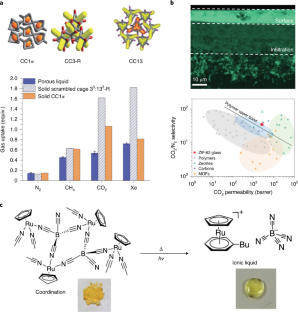
Abstract
Porous materials contain regions of empty space into which guest molecules can be selectively adsorbed and sometimes chemically transformed. This has made them useful in both industrial and domestic applications, ranging from gas separation, energy storage and ion exchange to heterogeneous catalysis and green chemistry. Porous materials are often ordered (crystalline) solids. Order—or uniformity—is frequently held to be advantageous, or even pivotal, to our ability to engineer useful properties in a rational way. Here we highlight the growing evidence that topological disorder can be useful in creating alternative properties in porous materials. In particular, we highlight here several concepts for the creation of novel porous liquids, rationalize routes to porous glasses and provide perspectives on applications for porous liquids and glasses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
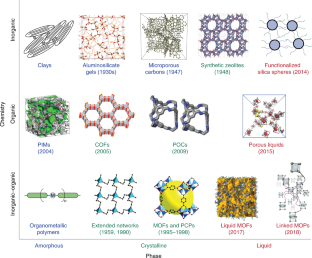
reproduced with permission from ref. 82, Springer Nature Ltd (PIMs); ref. 11, The American Association for the Advancement of Science (COFs); ref. 74, Springer Nature Ltd (porous liquids); ref. 83, John Wiley and Sons (extended networks); ref. 84, The American Association for the Advancement of Science (MOFs and PCPs); ref. 37, Springer Nature Ltd (liquid MOFs); ref. 26, John Wiley and Sons (functionalized silica spheres); ref. 16, under a Creative Commons license CC BY 4.0 (linked MOPs); ref. 85, Royal Society of Chemistry (aluminosilicate gels); ref. 86, AIP Publishing (microporous carbons); and adapted from ref. 73, Springer Nature Ltd (POCs)
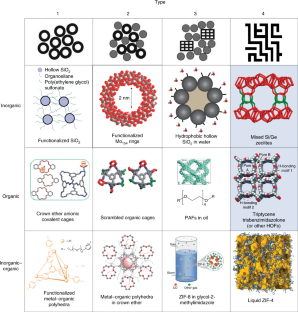
adapted with permission from ref. 25, John Wiley and Sons (schematics); ref. 28, John Wiley and Sons (O type 1); ref. 29, Springer Nature Ltd (IO type 1); ref. 30, American Chemical Society (I type 2); ref. 32, Royal Society of Chemistry (O type 2); ref. 87, John Wiley and Sons (IO type 2); ref. 33, American Chemical Society (I type 3); ref. 10, John Wiley and Sons, and ref. 53, under a Creative Commons license CC BY 4.0 (O type 3); ref. 34, under a Creative Commons license CC BY 4.0 (IO type 3); ref. 64, Royal Society of Chemistry (I type 4); ref. 65, John Wiley and Sons (O type 4); ref. 37, Springer Nature Ltd (IO type 4); and reproduced with permission from ref. 26, John Wiley and Sons (I type 1)
Similar content being viewed by others
References
Wright, P. A. The Chemistry of Microporous Framework Solids (Royal Society of Chemistry, 2008).
Davis, M. E. Ordered porous materials for emerging applications. Nature 417, 813–821 (2002).
Čejka, J., Morris, R. E. & Nachtigall, P. Zeolites in Catalysis: Properties and Applications (Catalysis Series Vol. 28, Royal Society of Chemistry, 2017).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal–organic frameworks. Science 341, 974–986 (2013).
Barrer, R. M. Syntheses and reactions of mordenite. J. Chem. Soc. https://doi.org/10.1039/JR9480002158 (1948).
Giannakoudakis, D. A. & Bandosz, T. Detoxification of Chemical Warfare Agents: from WWI to Multifunctional Nanocomposite Approaches (Springer, 2018).
Patrick, W. Silica gel and process of making same. US patent US1,297,724A (1919).
Yanagisawa, T., Shimizu, T. & Kuroda, K. The preparation of alkyltriinethylaininonium–kaneinite complexes and their conversion to microporous materials. Bull. Chem. Soc. Jpn 63, 988–992 (1990).
Budd, P. M. et al. Polymers of intrinsic microporosity (PIMs): robust, solution-processable, organic nanoporous materials. Chem. Commun. 2, 230–231 (2004).
Ben, T. et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 48, 9457–9460 (2009).
Cote, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Williams, K. A., Boydston, A. J. & Bielawski, C. W. Main-chain organometallic polymers: synthetic strategies, applications, and perspectives. Chem. Soc. Rev. 36, 729–744 (2007).
Honicke, I. M. et al. Balancing mechanical stability and ultrahigh porosity in crystalline framework materials. Angew. Chem. Int. Ed. 57, 13780–13783 (2018).
Cooper, A. I. Porous molecular solids and liquids. ACS Cent. Sci. 3, 544–553 (2017).
Ahmad, N., Younus, H. A., Chughtai, A. H. & Verpoort, F. Metal–organic molecular cages: applications of biochemical implications. Chem. Soc. Rev. 44, 9–25 (2015).
Carne-Sanchez, A. et al. Self-assembly of metal–organic polyhedra into supramolecular polymers with intrinsic microporosity. Nat. Commun. 9, 2506 (2018).
Kitagawa, S., Kitaura, R. & Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375 (2004).
Colon, Y. J. & Snurr, R. Q. High-throughput computational screening of metal–organic frameworks. Chem. Soc. Rev. 43, 5735–5749 (2014).
Simonov, A. & Goodwin, A. L. Designing disorder into crystalline materials. Nat. Rev. Chem. 4, 657–673 (2020).
Abednatanzi, S. et al. Mixed-metal metal–organic frameworks. Chem. Soc. Rev. 48, 2535–2565 (2019).
Besara, T. et al. Mechanism of the order–disorder phase transition, and glassy behavior in the metal–organic framework [(CH3)2NH2]Zn(HCOO)3. Proc. Natl Acad. Sci. USA 108, 6828–6832 (2011).
Sircar, S. & Golden, T. C. Purification of hydrogen by pressure swing adsorption. Sep. Sci. Technol. 35, 667–687 (2000).
Darunte, L. A., Oetomo, A. D., Walton, K. S., Sholl, D. S. & Jones, C. W. Direct air capture of CO2 using amine functionalized MIL-101(Cr). ACS Sustain. Chem. Eng. 4, 5761–5768 (2016).
Rochelle, G. T. Amine scrubbing for CO2 capture. Science 325, 1652–1654 (2009).
O’Reilly, N., Giri, N. & James, S. L. Porous liquids.Chem. Eur. J. 13, 3020–3025 (2007).
Zhang, J. S. et al. Porous liquids: a promising class of media for gas separation. Angew. Chem. Int. Ed. 54, 932–936 (2015).
Melaugh, G., Giri, N., Davidson, C. E., James, S. L. & Del Popolo, M. G. Designing and understanding permanent microporosity in liquids. Phys. Chem. Chem. Phys. 16, 9422–9431 (2014).
Jie, K. C. et al. Transforming porous organic cages into porous ionic liquids via a supramolecular complexation strategy. Angew. Chem. Int. Ed. 59, 2268–2272 (2020).
Ma, L. et al. Coordination cages as permanently porous ionic liquids. Nat. Chem. 12, 270–275 (2020).
Akutagawa, T. et al. Nanoscale assemblies of gigantic molecular {Mo-154}-rings: (dimethyldioctadecylammonium)20[Mo154O462H8(H2O)70]. Langmuir 24, 231–238 (2008).
Pierotti, R. A scaled particle theory of aqueous and nonaqueous solutions. Chem. Rev. 76, 717–726 (1976).
Kearsey, R. J., Alston, B., Briggs, M. E., Greenaway, R. L. & Cooper, A. I. Accelerated robotic discovery of type II porous liquids. Chem. Sci. 10, 9454–9465 (2019).
Moro, S., Parneix, C., Cabane, B., Sanson, N. & de Lacaillerie, J. B. D. Hydrophobization of silica nanoparticles in water: nanostructure and response to drying stress. Langmuir 33, 4709–4719 (2017).
Liu, H. et al. A hybrid absorption–adsorption method to efficiently capture carbon. Nat. Commun. 5, 4813 (2014).
Shan, W. et al. New class of type III porous liquids: a promising platform for rational adjustment of gas sorption behavior. ACS Appl. Mater. Interfaces 10, 32–36 (2018).
Tian, Y. Q. et al. The silica-like extended polymorphism of cobalt(ii) imidazolate three-dimensional frameworks: X-ray single-crystal structures and magnetic properties. Chem. Eur. J. 9, 5673–5685 (2003).
Gaillac, R. et al. Liquid metal–organic frameworks. Nat. Mater. 16, 1149–1154 (2017).
Ediger, M. D., Angell, C. A. & Nagel, S. R. Supercooled liquids and glasses. J. Phys. Chem. 100, 13200–13212 (1996).
Giri, N. et al. Alkylated organic cages: from porous crystals to neat liquids. Chem. Sci. 3, 2153–2157 (2012).
Hancock, B. C. & Zografi, G. The relationship between the glass-transition temperature and the water-content of amorphous pharmaceutical solids. Pharm. Res. 11, 471–477 (1994).
Angell, C. A. Liquid fragility and the glass transition in water and aqueous solutions. Chem. Rev. 102, 2627–2649 (2002).
Sare, E. J. & Angell, C. A. Glass-forming composition regions and glass transition temperature in nonaqueous electrolyte solutions. J. Solut. Chem. 2, 53–57 (1973).
Yang, K. et al. Predicting the Young’s modulus of silicate glasses using high-throughput molecular dynamics simulations and machine learning. Sci. Rep. 9, 8739 (2019).
Lasalle, A., Guizard, C., Maire, E., Adrien, J. & Deville, S. Particle redistribution and structural defect development during ice templating. Acta Mater. 60, 4594–4603 (2012).
Zhang, H. F. et al. Aligned two- and three-dimensional structures by directional freezing of polymers and nanoparticles. Nat. Mater. 4, 787–793 (2005).
Deville, S., Saiz, E., Nalla, R. K. & Tomsia, A. P. Freezing as a path to build complex composites. Science 311, 515–518 (2006).
McMillan, P. F. Polyamorphic transformations in liquids and glasses. J. Mater. Chem. 14, 1506–1512 (2004).
McMillan, P. F., Greaves, G. N., Wilson, M., Wilding, M. C. & Daisenberger, D. in Liquid Polymorphism (ed. Stanley, H. E.) 309–353 (Advances in Chemical Physics Vol. 152, Wiley, 2013).
Ha, A., Cohen, I., Zhao, X. L., Lee, M. & Kivelson, D. Supercooled liquids and polyamorphism. J. Phys. Chem. 100, 1–4 (1996).
Sporer, J. The Linde Solinox process: gypsum-free flue-gas desulphurization. Gas Sep. Purif. 6, 133–140 (1992).
Sholl, D. S. & Lively, R. P. Seven chemical separations to change the world. Nature 532, 435–437 (2016).
Greenaway, R. L. et al. Understanding gas capacity, guest selectivity, and diffusion in porous liquids. Chem. Sci. 8, 2640–2651 (2017).
Cahir, J. et al. Type 3 porous liquids based on non-ionic liquid phases—a broad and tailorable platform of selective, fluid gas sorbents. Chem. Sci. 11, 2077–2084 (2020).
Fu, Y. et al. Ultra-thin enzymatic liquid membrane for CO2 separation and capture. Nat. Commun. 9, 990 (2018).
Wang, Y. H. et al. A MOF glass membrane for gas separation. Angew. Chem. Int. Ed. 59, 4365–4369 (2020).
Whipple, D. T. & Kenis, P. J. A. Prospects of CO2 utilization via direct heterogeneous electrochemical reduction. J. Phys. Chem. Lett. 1, 3451–3458 (2010).
Kunene, T., Atifi, A. & Rosenthal, J. Selective CO2 reduction over Rose’s metal in the presence of an imidazolium ionic liquid electrolyte. ACS Appl. Energy Mater. 3, 4193–4200 (2020).
Wuttig, M. & Yamada, N. Phase-change materials for rewriteable data storage. Nat. Mater. 6, 824–832 (2007).
Funasako, Y., Mori, S. & Mochida, T. Reversible transformation between ionic liquids and coordination polymers by application of light and heat. Chem. Commun. 52, 6277–6279 (2016).
Wilmer, C. E. et al. Large-scale screening of hypothetical metal–organic frameworks. Nat. Chem. 4, 83–89 (2012).
Evans, J. D., Jelfs, K. E., Day, G. M. & Doonan, C. J. Application of computational methods to the design and characterisation of porous molecular materials. Chem. Soc. Rev. 46, 3286–3301 (2017).
Greenaway, R. et al. High-throughput discovery of organic cages and catenanes using computational screening fused with robotic synthesis. Nat. Commun. 9, 2849 (2018).
Pulido, A. et al. Functional materials discovery using energy–structure–function maps. Nature 543, 657–664 (2017).
Eliasova, P. et al. The ADOR mechanism for the synthesis of new zeolites. Chem. Soc. Rev. 44, 7177–7206 (2015).
Mastalerz, M. & Oppel, I. M. Rational construction of an extrinsic porous molecular crystal with an extraordinary high specific surface area. Angew. Chem. Int. Ed. 51, 5252–5255 (2012).
Ferlat, G. et al. van der Waals forces stabilize low-energy polymorphism in B2O3: implications for the crystallization anomaly. Phys. Rev. Mater. 3, 063603 (2019).
Zhang, W., Mazzarello, R., Wuttig, M. & Ma, E. Designing crystallization in phase-change materials for universal memory and neuro-inspired computing. Nat. Rev. Mater. 4, 150–168 (2019).
Tominaka, S. et al. Topochemical conversion of a dense metal–organic framework from a crystalline insulator to an amorphous semiconductor. Chem. Sci. 6, 1465–1473 (2015).
MacFarlane, D. R. et al. Energy applications of ionic liquids. Energy Environ. Sci. 7, 232–250 (2014).
Chen, X., Gao, H., Tang, Z. & Wang, G. Metal–organic framework-based phase change materials for thermal energy storage. Cell Rep. Phys. Sci. 1, 100218 (2020).
McGillicuddy, R. D., Thapa, S., Wenny, M. B., Gonzalez, M. I. & Mason, J. A. Metal–organic phase-change materials for thermal energy storage. J. Am. Chem. Soc. 142, 19170–19180 (2020).
Seo, S. et al. Phase-change ionic liquids for postcombustion CO2 capture. Energy Fuels 28, 5968–5977 (2014).
Tozawa, T. et al. Porous organic cages. Nat. Mater. 8, 973–978 (2009).
Giri, N. et al. Liquids with permanent porosity. Nature 527, 216–220 (2015).
Kinoshita, Y., Matsubara, I., Higuchi, T. & Saito, Y. The crystal structure of bis(adiponitrilo)copper(i) nitrate. Bull. Chem. Soc. Jpn 32, 1221–1226 (1959).
Hoskins, B. F. & Robson, R. Design and construction of a new class of scaffolding-like materials comprising infinite polymeric frameworks of 3D-linked molecular rods. A reappraisal of the Zn(CN)2 and Cd(CN)2 structures and the synthesis and structure of the diamond-related frameworks [N(CH3)4][Cu1Zn11(CN)4] and Cul[4,4′,4′′,4′′′-tetracyanotetraphenylmethane]BF4·xC6H5NO2. J. Am. Chem. Soc. 112, 1546–1554 (1990).
Yaghi, O. M., Li, G. M. & Li, H. L. Selective binding and removal of guests in a microporous metal–organic framework. Nature 378, 703–706 (1995).
Kondo, M., Yoshitomi, T., Seki, K., Matsuzaka, H. & Kitagawa, S. Three-dimensional framework with channeling cavities for small molecules: {[M2(4,4′-bpy)3(NO3)4]·xH2O}n (M=Co, Ni, Zn). Angew. Chem. Int. Ed. 36, 1725–1727 (1997).
Kistler, S. S. Coherent expanded aerogels and jellies. Nature 127, 741–741 (1931).
Bergaya, F. & Lagaly, G. Handbook of Clay Science (Elsevier, 2006).
Stoeckli, H. F. Microporous carbons and their characterization—the present state-of-the-art. Carbon 28, 1–6 (1990).
Song, Q. L. et al. Controlled thermal oxidative crosslinking of polymers of intrinsic microporosity towards tunable molecular sieve membranes. Nat. Commun. 5, 4813 (2014).
Izatt, R. M. Macrocyclic and Supramolecular Chemistry (Wiley, 2016).
Rosi, N. L. et al. Hydrogen storage in microporous metal–organic frameworks. Science 300, 1127–1129 (2003).
Comas-Vives, A. Amorphous SiO2 surface models: energetics of the dehydroxylation process, strain, ab initio atomistic thermodynamics and IR spectroscopic signatures. Phys. Chem. Chem. Phys. 18, 7475–7482 (2016).
Ranganathan, R. et al. Modeling high-temperature diffusion of gases in micro and mesoporous amorphous carbon. J. Chem. Phys. 143, 084701 (2015).
Deng, Z. et al. Facilitate gas transport through metal–organic polyhedra constructed porous liquid membrane. Small 16, 1907016 (2020).
Debenedetti, P. G. & Stillinger, F. H. Supercooled liquids and the glass transition. Nature 410, 259–267 (2001).
Widmer, R. N. et al. Pressure promoted low-temperature melting of metal–organic frameworks. Nat. Mater. 18, 370–376 (2019).
Ueda, T., Tominaga, T., Mochida, T., Takahashi, K. & Kimura, S. Photogeneration of microporous amorphous coordination polymers from organometallic ionic liquids. Chem. Eur. J. 24, 9490–9493 (2018).
Acknowledgements
T.D.B. acknowledges the Royal Society for a University Research Fellowship (UF150021), the Leverhulme Trust for a Philip Leverhulme Prize and the University of Canterbury Te Whare Wānanga o Waitaha, New Zealand, for a University of Cambridge Visiting Canterbury Fellowship. F.-X.C. acknowledges funding by the Agence Nationale de la Recherche (ANR-18-CE29-0009-01). A.I.C. acknowledges the Leverhulme Trust for funding through the Leverhulme Research Centre for Functional Materials Design.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Materials thanks Jorge Gascon, Russell Morris and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bennett, T.D., Coudert, FX., James, S.L. et al. The changing state of porous materials. Nat. Mater. 20, 1179–1187 (2021). https://doi.org/10.1038/s41563-021-00957-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-021-00957-w
This article is cited by
-
Analysis of metal–organic framework-based photosynthetic CO2 reduction
Nature Synthesis (2024)
-
Comparative study on hybrid-based MoS2-GO hybrid nanofluid flow over a three-dimensional extending surface: A numerical investigation
Colloid and Polymer Science (2024)
-
Comparative analysis of scaled entropies and topological properties of triphenylene-based metal and covalent organic frameworks
Chemical Papers (2024)
-
Materials for a changing planet
Nature Materials (2023)
-
Pore-engineered nanoarchitectonics for cancer therapy
NPG Asia Materials (2023)