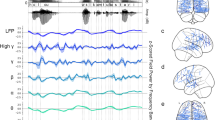
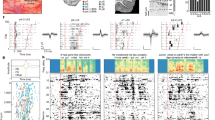
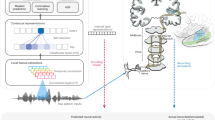
Abstract
The precise role of the human auditory cortex in representing speech sounds and transforming them to meaning is not yet fully understood. Here we used intracranial recordings from the auditory cortex of neurosurgical patients as they listened to natural speech. We found an explicit, temporally ordered and anatomically distributed neural encoding of multiple linguistic features, including phonetic, prelexical phonotactics, word frequency, and lexical–phonological and lexical–semantic information. Grouping neural sites on the basis of their encoded linguistic features revealed a hierarchical pattern, with distinct representations of prelexical and postlexical features distributed across various auditory areas. While sites with longer response latencies and greater distance from the primary auditory cortex encoded higher-level linguistic features, the encoding of lower-level features was preserved and not discarded. Our study reveals a cumulative mapping of sound to meaning and provides empirical evidence for validating neurolinguistic and psycholinguistic models of spoken word recognition that preserve the acoustic variations in speech.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Linguistic features were extracted from the SUBTLEX-US word frequency dataset59 and the English Lexicon Project website (https://elexicon.wustl.edu/). The data that support the findings of this study are available upon request from the corresponding author (N.M.). The data are shared upon request due to the sensitive nature of human patient data.
References
Chomsky, N. & Halle, M. The Sound Pattern of English (Harper & Row, 1968).
Vitevitch, M. S. & Luce, P. A. Probabilistic phonotactics and neighborhood activation in spoken word recognition. J. Mem. Lang. 40, 374–408 (1999).
Kiparsky, P. Word-formation and the lexicon. In Mid-America Linguistics Conference 3–29 (Mid-America Linguistics Conference, University of Kansas, Kansas, 1982).
Luce, P. A. & Pisoni, D. B. Recognizing spoken words: the neighborhood activation model. Ear Hear. 19, 1–36 (1998).
Buchanan, L., Westbury, C. & Burgess, C. Characterizing semantic space: neighborhood effects in word recognition. Psychon. Bull. Rev. 8, 531–544 (2001).
Grosjean, F. Spoken word recognition processes and the gating paradigm. Percept. Psychophys. 28, 267–283 (1980).
Marslen-Wilson, W. D. Speech shadowing and speech comprehension. Speech Commun. 4, 55–73 (1985).
Marslen-Wilson, W. D. Functional parallelism in spoken word-recognition. Cognition 25, 71–102 (1987).
Allopenna, P. D., Magnuson, J. S. & Tanenhaus, M. K. Tracking the time course of spoken word recognition using eye movements: evidence for continuous mapping models. J. Mem. Lang. 38, 419–439 (1998).
Dahan, D. & Magnuson, J. S. in Handbook of Psycholinguistics (eds Traxler, M. J. & Gernsbacher, M. A.) 249–283 (Elsevier, 2006); https://doi.org/10.1016/B978-012369374-7/50009-2
Magnuson, J. S., Mirman, D. & Harris, H. D. in The Cambridge Handbook of Psycholinguistics (eds Spivey, M. et al.) 76–103 (Cambridge Univ. Press, 2012); https://doi.org/10.1017/cbo9781139029377.008
Pisoni, D. B. & McLennan, C. T. in Neurobiology of Language (eds Hickok, G. & Small, S. L.) 239–253 (Elsevier, 2015); https://doi.org/10.1016/B978-0-12-407794-2.00020-1
Bidelman, G. M., Moreno, S. & Alain, C. Tracing the emergence of categorical speech perception in the human auditory system. NeuroImage 79, 201–212 (2013).
Fernald, A., Swingley, D. & Pinto, J. P. When half a word is enough: infants can recognize spoken words using partial phonetic information. Child Dev. 72, 1003–1015 (2001).
Magnuson, J. S., Dixon, J. A., Tanenhaus, M. K. & Aslin, R. N. The dynamics of lexical competition during spoken word recognition. Cogn. Sci. 31, 133–156 (2007).
Yee, E. & Sedivy, J. C. Eye movements to pictures reveal transient semantic activation during spoken word recognition. J. Exp. Psychol. Learn. Mem. Cogn. 32, 1–14 (2006).
Tyler, L. K., Voice, J. K. & Moss, H. E. The interaction of meaning and sound in spoken word recognition. Psychon. Bull. Rev. 7, 320–326 (2000).
Mirman, D. & Magnuson, J. S. Dynamics of activation of semantically similar concepts during spoken word recognition. Mem. Cogn. 37, 1026–1039 (2009). 2009 37:7.
McClelland, J. L. & Elman, J. L. The TRACE model of speech perception. Cogn. Psychol. 18, 1–86 (1986).
Scharenborg, O. Modeling the use of durational information in human spoken-word recognition. J. Acoust. Soc. Am. 127, 3758–3770 (2010).
Norris, D. Shortlist: a connectionist model of continuous speech recognition. Cognition 52, 189–234 (1994).
Scharenborg, O., Norris, D., ten Bosch, L. & McQueen, J. M. How should a speech recognizer work? Cogn. Sci. 29, 867–918 (2005).
Luce, P. A., Goldinger, S. D., Auer, E. T. & Vitevitch, M. S. Phonetic priming, neighborhood activation, and PARSYN. Percept. Psychophys. 62, 615–625 (2000).
Gaskell, M. G. & Marslen-Wilson, W. D. Integrating form and meaning: a distributed model of speech perception. Lang. Cogn. Process. 12, 613–656 (1997).
Norris, D. in Cognitive Models of Speech Processing: Psycholinguistic and Computational Perspectives (ed. Altmann, G. T. M.) 87–104 (MIT, 1990).
DeWitt, I. & Rauschecker, J. P. Phoneme and word recognition in the auditory ventral stream. Proc. Natl Acad. Sci. USA 109, E505–E514 (2012).
Poeppel, D. The neuroanatomic and neurophysiological infrastructure for speech and language. Curr. Opin. Neurobiol. 28, 142–149 (2014).
Price, C. J. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann. N. Y. Acad. Sci. 1191, 62–88 (2010).
de Heer, W. A., Huth, A. G., Griffiths, T. L., Gallant, J. L. & Theunissen, F. E. The hierarchical cortical organization of human speech processing. J. Neurosci. 37, 6539–6557 (2017).
Langers, D. R., Backes, W. H. & van Dijk, P. Spectrotemporal features of the auditory cortex: the activation in response to dynamic ripples. NeuroImage 20, 265–275 (2003).
Chan, A. M. et al. Speech-specific tuning of neurons in human superior temporal gyrus. Cereb. Cortex 24, 2679–2693 (2013).
Chang, E. F. et al. Categorical speech representation in human superior temporal gyrus. Nat. Neurosci. 13, 1428–1432 (2010).
Mesgarani, N., David, S. V., Fritz, J. B. & Shamma, S. A. Phoneme representation and classification in primary auditory cortex. J. Acoust. Soc. Am. 123, 899–909 (2008).
Steinschneider, M. et al. Differential activation of human core, non-core and auditory-related cortex during speech categorization tasks as revealed by intracranial recordings. Front. Neurosci 8, 240 (2014).
Ding, N., Melloni, L., Zhang, H., Tian, X. & Poeppel, D. Cortical tracking of hierarchical linguistic structures in connected speech. Nat. Neurosci. 19, 158–164 (2015).
Honey, C. J. et al. Slow cortical dynamics and the accumulation of information over long timescales. Neuron 76, 423–434 (2012).
Leonard, M. K., Bouchard, K. E., Tang, C. & Chang, E. F. Dynamic encoding of speech sequence probability in human temporal cortex. J. Neurosci. 35, 7203–7214 (2015).
Lerner, Y., Honey, C. J., Silbert, L. J. & Hasson, U. Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J. Neurosci. 31, 2906–2915 (2011).
Overath, T., McDermott, J. H., Zarate, J. M. & Poeppel, D. The cortical analysis of speech-specific temporal structure revealed by responses to sound quilts. Nat. Neurosci. 18, 903–911 (2015).
Caramazza, A., Berndt, R. S. & Basili, A. G. The selective impairment of phonological processing: a case study. Brain Lang. 18, 128–174 (1983).
Engelien, A. et al. The neural correlates of ‘deaf-hearing’ in man: conscious sensory awareness enabled by attentional modulation. Brain 123, 532–545 (2000).
Auerbach, S. H., Allard, T., Naeser, M., Alexander, M. P. & Albert, M. L. Pure word deafness: analysis of a case with bilateral lesions and a defect at the prephonemic level. Brain 105, 271–300 (1982).
Wang, E., Peach, R. K., Xu, Y., Schneck, M. & Manry, C. II Perception of dynamic acoustic patterns by an individual with unilateral verbal auditory agnosia. Brain Lang. 73, 442–455 (2000).
Poeppel, D. Pure word deafness and the bilateral processing of the speech code. Cogn. Sci. 25, 679–693 (2001).
Franklin, S., Turner, J., Ralph, M. A. L., Morris, J. & Bailey, P. J. A distinctive case of word meaning deafness? Cogn. Neuropsychol. 13, 1139–1162 (1996).
Boatman, D. et al. Transcortical sensory aphasia: revisited and revised. Brain 123, 1634–1642 (2000).
Kohn, S. E. & Friedman, R. B. Word-meaning deafness: a phonological–semantic dissociation. Cogn. Neuropsychol. 3, 291–308 (1986).
Rauschecker, J. P. & Scott, S. K. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat. Neurosci. 12, 718–724 (2009).
Rauschecker, J. P. in The Senses: A Comprehensive Reference (ed. Fritzsch, B.) 791–811(Elsevier, 2020).
Gaskell, M. G. & Marslen-Wilson, W. D. Representation and competition in the perception of spoken words. Cogn. Psychol. 45, 220–266 (2002).
Ray, S. & Maunsell, J. H. R. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 9, e1000610 (2011).
Buzsáki, G., Anastassiou, C. A. & Koch, C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 13, 407–420 (2012).
Clarke, S. & Morosan, P. in The Human Auditory Cortex (eds Poeppel, D., Overath, T., Popper, A. & Fay, R.) 11–38 (Springer, 2012).
Norman-Haignere, S. V. & McDermott, J. H. Neural responses to natural and model-matched stimuli reveal distinct computations in primary and nonprimary auditory cortex. PLoS Biol. 16, e2005127 (2018).
Baumann, S., Petkov, C. I. & Griffiths, T. D. A unified framework for the organization of the primate auditory cortex. Front. Syst. Neurosci. 0, 11 (2013).
Shaoul, C. & Westbury, C. Exploring lexical co-occurrence space using HiDEx. Behav. Res. Methods 42, 393–413 (2010).
Ladefoged, P. & Johnson, K. A Course in Phonetics (Wadsworth Publishing Company,2010).
Mesgarani, N., Cheung, C., Johnson, K. & Chang, E. F. Phonetic feature encoding in human superior temporal gyrus. Science 343, 1006–1010 (2014).
Brysbaert, M. & New, B. Moving beyond Kučera and Francis: a critical evaluation of current word frequency norms and the introduction of a new and improved word frequency measure for American English. Behav. Res. Methods 41, 977–990 (2009).
Ylinen, S. et al. Predictive coding of phonological rules in auditory cortex: a mismatch negativity study. Brain Lang. 162, 72–80 (2016).
Friston, K. The free-energy principle: a rough guide to the brain? Trends Cogn. Sci. 13, 293–301 (2009).
Gagnepain, P., Henson, R. N. & Davis, M. H. Temporal predictive codes for spoken words in auditory cortex. Curr. Biol. 22, 615–621 (2012).
Brodbeck, C., Hong, L. E. & Simon, J. Z. Rapid transformation from auditory to linguistic representations of continuous speech. Curr. Biol. 28, 3976–3983 (2018).
Dahan, D., Magnuson, J. S. & Tanenhaus, M. K. Time course of frequency effects in spoken-word recognition: evidence from eye movements. Cogn. Psychol. 42, 317–367 (2001).
Marslen-Wilson, W. D. & Welsh, A. Processing interactions and lexical access during word recognition in continuous speech. Cogn. Psychol. 10, 29–63 (1978).
Balling, L. W. & Baayen, R. H. Probability and surprisal in auditory comprehension of morphologically complex words. Cognition 125, 80–106 (2012).
Wurm, L. H., Ernestus, M., Schreuder, R. & Baayen, R. H. Dynamics of the auditory comprehension of prefixed words. Ment. Lex. 1, 125–146 (2006).
Balota, D. A. et al. The English Lexicon Project. Behav. Res. Methods 39, 445–459 (2007).
Danguecan, A. N. & Buchanan, L. Semantic neighborhood effects for abstract versus concrete words. Front. Psychol. 7, 1034 (2016).
Mirman, D. & Magnuson, J. S. The impact of semantic neighborhood density on semantic access. In Proc. 28th Annual Conference of the Cognitive Science Society (eds Sun, R. & Miyake, N.) 1823–1828 (2006).
Broderick, M. P., Anderson, A. J., di Liberto, G. M., Crosse, M. J. & Lalor, E. C. Electrophysiological correlates of semantic dissimilarity reflect the comprehension of natural, narrative speech. Curr. Biol. 28, 803–809.e3 (2018).
di Liberto, G. M., O’Sullivan, J. A. & Lalor, E. C. Low-frequency cortical entrainment to speech reflects phoneme-level processing. Curr. Biol. 25, 2457–2465 (2015).
Di Liberto, G. M., Wong, D., Melnik, G. A. & de Cheveigné, A. Low-frequency cortical responses to natural speech reflect probabilistic phonotactics. NeuroImage 196, 237–247 (2019).
Yang, X.-B., Wang, K. & Shamma, S. A. Auditory representations of acoustic signals. IEEE Trans. Inf. Theory 38, 824–839 (1992).
Kluender, K. R., Coady, J. A. & Kiefte, M. Sensitivity to change in perception of speech. Speech Commun. 41, 59–69 (2003).
Daube, C., Ince, R. A. A. & Gross, J. Simple acoustic features can explain phoneme-based predictions of cortical responses to speech. Curr. Biol. 29, 1924–1937 (2019).
Fischl, B. et al. Automatically parcellating the human cerebral cortex. Cereb. Cortex 14, 11–22 (2004).
Pisoni, D. B. in Talker Variability in Speech Processing (eds Johnson, K. & Mullennix, J. W.) 9–32 (Morgan Kaufmann, 1997).
Luce, P. A. & McLennan, C. T. in The Handbook of Speech Perception (eds Pisoni, D. B. & Remez, R. E.) 590–609 (Blackwell, 2008); https://doi.org/10.1002/9780470757024.ch24
Port, R. F. Rich memory and distributed phonology. Lang. Sci. 32, 43–55 (2010).
Nosofsky, R. M. Attention, similarity, and the identification–categorization relationship. J. Exp. Psychol. Gen. 115, 39–57 (1986).
Kruschke, J. K. ALCOVE: an exemplar-based connectionist model of category learning. Psychol. Rev. 99, 22–44 (1992).
Magnuson, J. S., Nusbaum, H. C., Akahane-Yamada, R. & Saltzman, D. Talker familiarity and the accommodation of talker variability. Atten. Percept. Psychophys. 83, 1842–1860 (2021).
McLaughlin, D., Dougherty, S., Lember, R. & Perrachione, T. Episodic memory for words enhances the language familiarity effect in talker identification. In Proc. 18th International Congress of Phonetic Sciences (ed. The Scottish Consortium for ICPhS 2015) 367.1-4 (University of Glasgow, Glasgow, 2015).
Choi, J. Y., Hu, E. R. & Perrachione, T. K. Varying acoustic–phonemic ambiguity reveals that talker normalization is obligatory in speech processing. Atten. Percept. Psychophys. 80, 784–797 (2018).
Pisoni, D. B. & Levi, S. V. in The Oxford Handbook of Psycholinguistics (ed. Gaskell, M. G.) 3–18 (Oxford Univ. Press, 2007); https://doi.org/10.1093/oxfordhb/9780198568971.013.0001
Vitevitch, M. S., Luce, P. A., Pisoni, D. B. & Auer, E. T. Phonotactics, neighborhood activation, and lexical access for spoken words. Brain Lang. 68, 306–311 (1999).
von Economo, C. F. & Koskinas, G. N. Die Cytoarchitektonik der Hirnrinde des Erwachsenen Menschen (J. Springer, 1925).
Galaburda, A. & Sanides, F. Cytoarchitectonic organization of the human auditory cortex. J. Comp. Neurol. 190, 597–610 (1980).
Morosan, P., Rademacher, J., Palomero-Gallagher, N. & Zilles, K. in The Auditory Cortex (eds Heil, P., Scheich, H., Budinger, E. & Konig, R.) 45–68 (Psychology Press, 2005).
Hopf, A. Die Myeloarchitektonik des Isocortex Temporalis Beim Menschen (De Gruyter, 1951).
Moerel, M., De Martino, F. & Formisano, E. An anatomical and functional topography of human auditory cortical areas. Front. Neurosci. 8, 225 (2014).
Nourski, K. V. Auditory processing in the human cortex: an intracranial electrophysiology perspective. Laryngoscope Investig. Otolaryngol 2, 147–156 (2017).
Griffiths, T. D. & Warren, J. D. The planum temporale as a computational hub. Trends Neurosci. 25, 348–353 (2002).
Hillis, A. E., Rorden, C. & Fridriksson, J. Brain regions essential for word comprehension: drawing inferences from patients. Ann. Neurol. 81, 759–768 (2017).
Mesulam, M.-M. et al. Word comprehension in temporal cortex and Wernicke area. Neurology 92, e224–e233 (2019).
Binder, J. R. Current controversies on Wernicke’s area and its role in language. Curr. Neurol. Neurosci. Rep. 17, 58 (2017).
Muller, L., Hamilton, L. S., Edwards, E., Bouchard, K. E. & Chang, E. F. Spatial resolution dependence on spectral frequency in human speech cortex electrocorticography. J. Neural Eng. 13, 56013 (2016).
Khodagholy, D. et al. NeuroGrid: recording action potentials from the surface of the brain. Nat. Neurosci. 18, 310–315 (2015).
Blumstein, S. E., Baker, E. & Goodglass, H. Phonological factors in auditory comprehension in aphasia. Neuropsychologia 15, 19–30 (1977).
Norris, D., McQueen, J. M. & Cutler, A. Prediction, Bayesian inference and feedback in speech recognition. Lang. Cogn. Neurosci. 31, 4–18 (2016).
Magnuson, J. S., Mirman, D., Luthra, S., Strauss, T. & Harris, H. D. Interaction in spoken word recognition models: feedback helps. Front. Psychol. 9, 369 (2018).
Norris, D. & McQueen, J. M. Shortlist B: a Bayesian model of continuous speech recognition. Psychol. Rev. 115, 357–395 (2008).
Hamilton, L. S., Oganian, Y., Hall, J. & Chang, E. F. Parallel and distributed encoding of speech across human auditory cortex. Cell 184, 4626–4639.e13 (2021).
Groppe, D. M. et al. iELVis: an open source MATLAB toolbox for localizing and visualizing human intracranial electrode data. J. Neurosci. Methods 281, 40–48 (2017).
Jenkinson, M. & Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 5, 143–156 (2001).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17, 825–841 (2002).
Smith, S. M. Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155 (2002).
Papademetris, X. et al. BioImage Suite: an integrated medical image analysis suite: an update. Insight J. 2006, 209 (2006).
Sweet, R. A., Dorph‐Petersen, K. & Lewis, D. A. Mapping auditory core, lateral belt, and parabelt cortices in the human superior temporal gyrus. J. Comp. Neurol. 491, 270–289 (2005).
Ozker, M., Schepers, I. M., Magnotti, J. F., Yoshor, D. & Beauchamp, M. S. A double dissociation between anterior and posterior superior temporal gyrus for processing audiovisual speech demonstrated by electrocorticography. J. Cogn. Neurosci. 29, 1044–1060 (2017).
Gorman, K., Howell, J. & Wagner, M. Prosodylab-Aligner: a tool for forced alignment of laboratory speech. Can. Acoust. 39, 192–193 (2011).
Crosse, M. J., di Liberto, G. M., Bednar, A. & Lalor, E. C. The Multivariate Temporal Response Function (mTRF) toolbox: a MATLAB toolbox for relating neural signals to continuous stimuli. Front. Hum. Neurosci. 10, 604 (2016).
Ward, J. H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 58, 236–244 (1963).
Acknowledgements
This work was funded by National Institutes of Health grant no. R01DC018805 (N.M.) and National Institute on Deafness and Other Communication Disorders grant no. R01DC014279 (N.M.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
M.K. and N.M. designed the experiment. M.K., S.A., J.H., S.B., A.D.M. and N.M. recorded the neural data. M.K. and N.M. analysed the data and wrote the manuscript. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Human Behaviour thanks Frederic Theunissen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Electrode locations.
Electrodes are distributed across fifteen subjects and are either depth or subdural grids Cand/or strips. Shape indicates electrode type, where circles represent depth electrodes, and triangles represent subdural contacts. Shape colour indicates which of the fifteen subjects an electrode belongs to.
Extended Data Fig. 2 Speech-responsiveness.
Circles represent electrodes in the immediate vicinity of the auditory cortex, based on their 3D coordinates in the ‘fsaverage’ space. Filled circles indicate speech-responsiveness, meaning the electrode site responds significantly differently to speech compared to silence (see ‘Electrode selection’ in Methods). Non-responsiveness could mean the electrode is not sound-responsive, is sound-responsive but not speech-responsive, or has insufficient signal-to-noise ratio (SNR). The non-responsive electrodes were excluded from all analyses.
Extended Data Fig. 3 Phonetic features.
Each phoneme is represented by 22 binary features based on its voicing, place, and manner of articulation features.
Extended Data Fig. 4 Selecting stimulus window length for prediction.
A window size of 510 ms was chosen to maximize linear model performance with the minimal number of parameters. We fit multiple models, each with a different number of time-lags (window size), from 60 ms to 760 ms. Each model was trained with the full list of predictors shown in Fig. 1c on all electrodes selected by the selection criteria described in Methods (n = 242), and only differed from the other models in the number of lags. Error bars indicate standard error (SE) over electrodes. To compare two different sizes, we perform a paired-sample one-tailed t-test on the cross-validated out-of-sample prediction r-values to determine whether the larger model improves upon the smaller one. The 510 ms model (dashed line) showed a significant improvement over all smaller models (60 ms – 410 ms, p < 0.001; 460 ms, p = 0.023). No larger model showed significant improvement over the 510 ms model (p > 0.5).
Extended Data Fig. 5 Correlations among features.
The linguistic features defined in this study are themselves correlated with each other. This plot shows the absolute value of the Pearson correlation coefficient for all pairs of 1-dimensional linguistic features (22-dimensional phonetic features excluded from figure; L1: lexical entropy, L2: lexical surprisal). The correlations are computed on the same 30-minute dataset used for all other analyses. All correlations are statistically significant (p < 0.001).
Extended Data Fig. 6 Explained variance of TRF diversity.
The bootstrapped (n = 1000) PCA analysis in Fig. 3 generates multiple eigenvectors at each bootstrap sample. We use the eigenvector that captures the most variance for computing the time courses (3a) and peak latencies (3b). This plot shows the mean and standard deviation of the explained variance for each of the top-10 principal components, computed using the same bootstrap procedure. In all cases, the first principal component captures roughly half of the total variance across all electrodes.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keshishian, M., Akkol, S., Herrero, J. et al. Joint, distributed and hierarchically organized encoding of linguistic features in the human auditory cortex. Nat Hum Behav 7, 740–753 (2023). https://doi.org/10.1038/s41562-023-01520-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-023-01520-0
This article is cited by
-
The language network as a natural kind within the broader landscape of the human brain
Nature Reviews Neuroscience (2024)
-
Emergence of the cortical encoding of phonetic features in the first year of life
Nature Communications (2023)