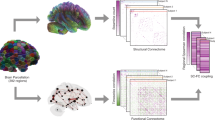
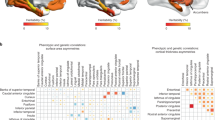
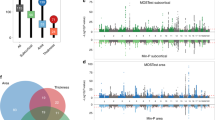
Abstract
Broca reported ~150 years ago that particular lesions of the left hemisphere impair speech. Since then, other brain regions have been reported to show lateralized structure and function. Yet, studies of brain asymmetry have limited their focus to pairwise comparisons between homologous regions. Here, we characterized separable whole-brain asymmetry patterns in grey and white matter structure from n = 37,441 UK Biobank participants. By pooling information on left–right shifts underlying whole-brain structure, we deconvolved signatures of brain asymmetry that are spatially distributed rather than locally constrained. Classically asymmetric regions turned out to belong to more than one asymmetry pattern. Instead of a single dominant signature, we discovered complementary asymmetry patterns that contributed similarly to whole-brain asymmetry at the population level. These asymmetry patterns were associated with unique collections of phenotypes, ranging from early lifestyle factors to demographic status to mental health indicators.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The UKBB data are available to other investigators online (ukbiobank.ac.uk). https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases. Source data are provided with this paper.
Code availability
A full collection of asymmetry patterns is freely available to and open for reuse by the reader at: https://github.com/ksaltoun/Dissociable_Asymmetry_Patterns. The analysis scripts that reproduce the results of the present study are available upon request.
References
Broca, P. Remarques sur le siège de la faculté du langage articulé, suivies d’une observation d’aphémie (perte de la parole). Bull. Memoires Soc. Anatomique Paris 6, 330–357 (1861).
Gazzaniga, M. S. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain 123, 1293–1326 (2000).
Toga, A. W. & Thompson, P. M. Mapping brain asymmetry. Nat. Rev. Neurosci. 4, 37–48 (2003).
Hartwigsen, G., Bengio, Y. & Bzdok, D. How does hemispheric specialization contribute to human-defining cognition? Neuron 109, 2075–2090 (2021).
Pinker, S. & Jackendoff, R. The faculty of language: what’s special about it? Cognition 95, 201–236 (2005).
Tomasello, M., Carpenter, M., Call, J., Behne, T. & Moll, H. Understanding and sharing intentions: the origins of cultural cognition. Behav. Brain Sci. 28, 675–691 (2005).
Gunturkun, O. & Ocklenburg, S. Ontogenesis of lateralization. Neuron 94, 249–263 (2017).
Vallortigara, G. The evolutionary psychology of left and right: costs and benefits of lateralization. Dev. Psychobiol. 48, 418–427 (2006).
Rogers, L. J., Zucca, P. & Vallortigara, G. Advantages of having a lateralized brain. Proc. Biol. Sci. 271, S420–S422 (2004).
Dadda, M., Domenichini, A., Piffer, L., Argenton, F. & Bisazza, A. Early differences in epithalamic left-right asymmetry influence lateralization and personality of adult zebrafish. Behav. Brain Res. 206, 208–215 (2010).
Vallortigara, G. Comparative neuropsychology of the dual brain: a stroll through animals’ left and right perceptual worlds. Brain Lang. 73, 189–219 (2000).
Vallortigara, G. & Rogers, L. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 28, 575–589 (2005).
Ringo, J. L., Doty, R. W., Demeter, S. & Simard, P. Y. Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb. Cortex 4, 331–343 (1994).
Kong, X. Z. et al. Large-scale phenomic and genomic analysis of brain asymmetrical skew. Cereb. Cortex 31, 4151–4168 (2021).
Xiang, L., Crow, T. & Roberts, N. Cerebral torque is human specific and unrelated to brain size. Brain Struct. Funct. 224, 1141–1150 (2019).
Zhao, L., Matloff, W., Shi, Y., Cabeen, R. P. & Toga, A. W. Mapping complex brain torque components and their genetic architecture and phenomic associations in 24,112 individuals. Biol. Psychiatry 91, 753–768 (2022).
Li, X., Crow, T. J., Hopkins, W. D., Gong, Q. & Roberts, N. Human torque is not present in chimpanzee brain. Neuroimage 165, 285–293 (2018).
Bear, D., Schiff, D., Saver, J., Greenberg, M. & Freeman, R. Quantitative analysis of cerebral asymmetries. Fronto-occipital correlation, sexual dimorphism and association with handedness. Arch. Neurol. 43, 598–603 (1986).
Barrick, T. R. et al. Automatic analysis of cerebral asymmetry: an exploratory study of the relationship between brain torque and planum temporale asymmetry. Neuroimage 24, 678–691 (2005).
Shapleske, J., Rossell, S. L., Woodruff, P. W. & David, A. S. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res. Brain Res. Rev. 29, 26–49 (1999).
Nakada, T., Fujii, Y., Yoneoka, Y. & Kwee, I. L. Planum temporale: where spoken and written language meet. Eur. Neurol. 46, 121–125 (2001).
Middleton, F. A. & Strick, P. L. Cerebellar projections to the prefrontal cortex of the primate. J. Neurosci. 21, 700–712 (2001).
Palesi, F. et al. Contralateral cortico-ponto-cerebellar pathways reconstruction in humans in vivo: implications for reciprocal cerebro-cerebellar structural connectivity in motor and non-motor areas. Sci. Rep. 7, 12841 (2017).
Neubauer, S., Gunz, P., Scott, N. A., Hublin, J. J. & Mitteroecker, P. Evolution of brain lateralization: a shared hominid pattern of endocranial asymmetry is much more variable in humans than in great apes. Sci. Adv. 6, eaax9935 (2020).
Hou, L. et al. Measurement of sylvian fissure asymmetry and occipital bending in humans and pan troglodytes. Neuroimage 184, 855–870 (2019).
Shaywitz, B. A. et al. Sex differences in the functional organization of the brain for language. Nature 373, 607–609 (1995).
Voyer, D. On the magnitude of laterality effects and sex differences in functional lateralities. Laterality 1, 51–83 (1996).
Voyer, D., Voyer, S. & Bryden, M. P. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol. Bull. 117, 250–270 (1995).
Chance, S. A. & Crow, T. J. Distinctively human: cerebral lateralisation and language in Homo sapiens. J. Anthropol. Sci. 85, 83–100 (2007).
Badzakova-Trajkov, G., Häberling, I. S., Roberts, R. P. & Corballis, M. C. Cerebral asymmetries: complementary and independent processes. PLoS ONE 5, e9682 (2010).
Corballis, M. C. Language evolution: a changing perspective. Trends Cogn. Sci. 21, 229–236 (2017).
Tomasello, M. The human adaptation for culture. Annu. Rev. Anthropol. 28, 509–529 (1999).
Vaesen, K. The cognitive bases of human tool use. Behav. Brain Sci. 35, 203–218 (2012).
Tomasello, M. & Call, J. Thirty years of great ape gestures. Anim. Cogn. 22, 461–469 (2019).
Thibault, S. et al. Tool use and language share syntactic processes and neural patterns in the basal ganglia. Science 374, eabe0874 (2021).
Muret, D. et al. Touch improvement at the hand transfers to the face. Curr. Biol. 24, R736–R737 (2014).
Vyas, S. et al. Neural population dynamics underlying motor learning transfer. Neuron 97, 1177–1186 e1173 (2018).
Dahlin, E., Neely, A. S., Larsson, A., Backman, L. & Nyberg, L. Transfer of learning after updating training mediated by the striatum. Science 320, 1510–1512 (2008).
Sha, Z. et al. Handedness and its genetic influences are associated with structural asymmetries of the cerebral cortex in 31,864 individuals. Proc. Natl Acad. Sci. USA 118, e2113095118 (2021).
Vingerhoets, G. Phenotypes in hemispheric functional segregation? Perspectives and challenges. Phys. Life Rev. 30, 1–18 (2019).
Chari, T., Banerjee, J. & Pachter, L. The specious art of single-cell genomics. Preprint at bioRxiv https://doi.org/10.1101/2021.08.25.457696 (2021).
Alfaro-Almagro, F. et al. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage 166, 400–424 (2018).
Miller, K. L. et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 19, 1523–1536 (2016).
Herbert, M. R. et al. Abnormal asymmetry in language association cortex in autism. Ann. Neurol. 52, 588–596 (2002).
Steinmetz, H. Structure, functional and cerebral asymmetry: in vivo morphometry of the planum temporale. Neurosci. Biobehav. Rev. 20, 587–591 (1996).
Spreng, R. N. et al. The default network of the human brain is associated with perceived social isolation. Nat. Commun. 11, 1–11 (2020).
Schurz, M. et al. Variability in brain structure and function reflects lack of peer support. Cereb. Cortex 31, 4612–4627 (2021).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. https://doi.org/10.1038/nbt.4314 (2018).
Diaz-Papkovich, A., Anderson-Trocmé, L., Ben-Eghan, C. & Gravel, S. UMAP reveals cryptic population structure and phenotype heterogeneity in large genomic cohorts. PLoS Genet. 15, e1008432 (2019).
McInnes, L., Healy, J. & Melville, J. Umap: uniform manifold approximation and projection for dimension reduction. Preprint at https://arxiv.org/abs/1802.03426 (2018).
Gerbrands, J. J. On the relationships between SVD, KLT and PCA. Pattern Recognit. 14, 375–381 (1981).
Paul, L. K. et al. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat. Rev. Neurosci. 8, 287–299 (2007).
Kuhn, H. W. The Hungarian method for the assignment problem. Naval Res. Logist. Q. 2, 83–97 (1955).
Millard, L. A. C., Davies, N. M., Gaunt, T. R., Davey Smith, G. & Tilling, K. Software application profile: PHESANT: a tool for performing automated phenome scans in UK Biobank. Int. J. Epidemiol. 47, 29–35 (2018).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57, 289–300 (1995).
Sha, Z. et al. The genetic architecture of structural left-right asymmetry of the human brain. Nat. Hum. Behav. 5, 1226–1239 (2021).
Raizada, R. D., Richards, T. L., Meltzoff, A. & Kuhl, P. K. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. Neuroimage 40, 1392–1401 (2008).
Genovese, C. R., Lazar, N. A. & Nichols, T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15, 870–878 (2002).
Acknowledgements
This study was supported by the Brain Canada Foundation, through the Canada Brain Research Fund, with the financial support of Health Canada, National Institutes of Health (grant nos. NIH R01 AG068563A and NIH R01 R01DA053301-01A1 to D.B.), the Canadian Institute of Health Research (grant nos. CIHR 438531 and CIHR 470425 to D.B.), the Healthy Brains Healthy Lives initiative (Canada First Research Excellence fund to D.B.), Google (Research Award, Teaching Award to D.B.) and by the CIFAR Artificial Intelligence Chairs programme (Canada Institute for Advanced Research to D.B.).
The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
K.S. and D.B. conceived, executed and wrote the paper. All authors analysed the data and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Human Behaviour thanks Xiang-Zhen Kong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7 and Table 1.
Supplementary Data Fig. 1
Statistical source data of UMAP stability test.
Supplementary Data Fig. 2
Statistical source data of explained variance permutation tests; feature contribution of top 25 asymmetry patterns.
Supplementary Data Fig. 3
Statistical source data of bootstrap robustness test + permutation testing and explained variance permutation tests.
Supplementary Data Fig. 4
Comparison of number of phenotypic hits on a regional versus asymmetry pattern-based approach.
Supplementary Data Fig. 5
SVD-based non-parametric permutation test for consistent handedness-based asymmetry pattern deviations.
Supplementary Data Fig. 6
SVD-based non-parametric permutation test for consistent sex-based asymmetry pattern deviations.
Supplementary Data Fig. 7
SVD-Based non-parametric permutation test for consistent IQ-based asymmetry pattern deviations.
Supplementary Data Table 1
Effect of sex and age on asymmetry pattern expression ANOVA results.
Source data
Source Data Fig. 1
UMAP 1+2 coordinates; absolute average lateralization index.
Source Data Fig. 2
Asymmetry pattern 1 feature contributions; Manhattan plot; age-sex graph; representative brain feature measures.
Source Data Fig. 3
Asymmetry pattern 2 feature contributions; Manhattan plot; age-sex graph; representative brain feature measures.
Source Data Fig. 4
Asymmetry pattern 3 feature contributions; Manhattan plot; age-sex graph; representative brain feature measures.
Source Data Fig. 5
Asymmetry pattern 4 feature contributions; Manhattan plot; age-sex graph; representative brain feature measures.
Source Data Fig. 6
Regional non-parametric permutation test for consistent handedness-based asymmetry deviations.
Source Data Fig. 7
Regional non-parametric permutation test for consistent sex-based asymmetry deviations.
Source Data Fig. 8
Regional non-parametric permutation test for consistent IQ-based asymmetry deviations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saltoun, K., Adolphs, R., Paul, L.K. et al. Dissociable brain structural asymmetry patterns reveal unique phenome-wide profiles. Nat Hum Behav 7, 251–268 (2023). https://doi.org/10.1038/s41562-022-01461-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-022-01461-0
This article is cited by
-
Brain asymmetries from mid- to late life and hemispheric brain age
Nature Communications (2024)
-
Longitudinal microstructural changes in 18 amygdala nuclei resonate with cortical circuits and phenomics
Communications Biology (2024)
-
Language network lateralization is reflected throughout the macroscale functional organization of cortex
Nature Communications (2023)