Abstract
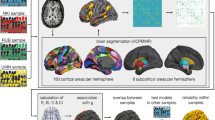
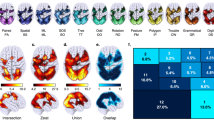
The human brain network is modular—consisting of communities of tightly interconnected nodes1. This network contains local hubs, which have many connections within their own communities, and connector hubs, which have connections diversely distributed across communities2,3. A mechanistic understanding of these hubs and how they support cognition has not been demonstrated. Here, we leveraged individual differences in hub connectivity and cognition. We show that a model of hub connectivity accurately predicts the cognitive performance of 476 individuals in 4 distinct tasks. Moreover, there is a general optimal network structure for cognitive performance—individuals with diversely connected hubs and consequent modular brain networks exhibit increased cognitive performance, regardless of the task. Critically, we find evidence consistent with a mechanistic model in which connector hubs tune the connectivity of their neighbours to be more modular while allowing for task appropriate information integration across communities, which increases global modularity and cognitive performance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bertolero, M. A., Yeo, B. T. T. & D’Esposito, M. The modular and integrative functional architecture of the human brain. Proc. Natl Acad. Sci. USA 112, E6798–E6807 (2015).
Bertolero, M. A., Yeo, B. T. T. & D’Esposito, M. The diverse club. Nat. Commun. 8, 1277 (2017).
Guimerà, R., Guimera, R., Amaral, L. A. N. & Amaral, L. Functional cartography of complex metabolic networks. Nature 433, 895–900 (2005).
Meunier, D., Lambiotte, R. & Bullmore, E. T. Modular and hierarchically modular organization of brain networks. Front. Neurosci. 4, 200 (2010).
Bassett, D. S. et al. Efficient physical embedding of topologically complex information processing networks in brains and computer circuits. PLoS Comput. Biol. 6, e1000748 (2010).
Laughlin, S. B., de Ruyter van Steveninck, R. R. & Anderson, J. C. The metabolic cost of neural information. Nat. Neurosci. 1, 36–41 (1998).
Lord, L.-D., Expert, P., Huckins, J. F. & Turkheimer, F. E. Cerebral energy metabolism and the brain’s functional network architecture: an integrative review. J. Cereb. Blood Flow Metab. 33, 1347–1354 (2013).
Raichle, M. E. & Gusnard, D. A. Appraising the brain’s energy budget. Proc. Natl Acad. Sci. USA 99, 10237–10239 (2002).
Harris, J. J. & Attwell, D. The energetics of CNS white matter. J. Neurosci. 32, 356–371 (2012).
Kuzawa, C. W. et al. Metabolic costs and evolutionary implications of human brain development. Proc. Natl Acad. Sci. USA 111, 13010–13015 (2014).
Krienen, F. M., Yeo, B. T. T., Ge, T., Buckner, R. L. & Sherwood, C. C. Transcriptional profiles of supragranular-enriched genes associate with corticocortical network architecture in the human brain. Proc. Natl Acad. Sci. USA 113, E469–E478 (2016).
Hawrylycz, M. et al. Canonical genetic signatures of the adult human brain. Nat. Neurosci. 18, 1832–1844 (2015).
Wagner, G. P. & Zhang, J. The pleiotropic structure of the genotype-phenotype map: the evolvability of complex organisms. Nat. Rev. Genet. 12, 204–213 (2011).
Clune, J., Mouret, J.-B. & Lipson, H. The evolutionary origins of modularity. Proc. Biol. Sci. 280, 1–9 (2013).
Kashtan, N., Kashtan., Alon, U. & Alon, U. Spontaneous evolution of modularity and network motifs. Proc. Natl Acad. Sci. USA 102, 13773–13778 (2005).
Simon, H. A. in Facets of Systems Science International Federation for Systems Research International Series on Systems Science and Engineering, Vol. 7 457–476 (Springer, Boston, MA, 1991).
Fodor, J. The Modularity of Mind (MIT Press, Cambridge, MA, 1983).
Coltheart, M. Modularity and cognition. Trends Cogn. Sci. 3, 115–120 (1999).
Robinson, P. A., Henderson, J. A., Matar, E., Riley, P. & Gray, R. T. Dynamical reconnection and stability constraints on cortical network architecture. Phys. Rev. Lett. 103, 108104 (2009).
Clune, J., Mouret, J.-B. & Lipson, H. The evolutionary origins of modularity. Proc. Biol. Sci. 280, 20122863 (2013).
Tosh, C. R., Tosh, C. R., McNally, L. & McNally, L. The relative efficiency of modular and non-modular networks of different size. Proc. Biol. Sci. 282, 20142568 (2015).
Stevens, A. A., Tappon, S. C., Garg, A. & Fair, D. A. Functional brain network modularity captures inter- and intra-individual variation in working memory capacity. PLoS ONE 7, e30468 (2012).
Arnemann, K. L. et al. Functional brain network modularity predicts response to cognitive training after brain injury. Neurology 84, 1568–1574 (2015).
Gallen, C. L. et al. Modular brain network organization predicts response to cognitive training in older adults. PLoS ONE. 11, e0169015 (2016).
Warren, D. E. et al. Network measures predict neuropsychological outcome after brain injury. Proc. Natl Acad. Sci. USA 111, 14247–14252 (2014).
Gratton, C., Nomura, E. M., Pérez, F. & D’Esposito, M. Focal brain lesions to critical locations cause widespread disruption of the modular organization of the brain. J. Cogn. Neurosci. 24, 1275–1285 (2012).
van den Heuvel, M. P. & Sporns, O. Network hubs in the human brain. Trends Cogn. Sci. 17, 683–696 (2013).
Rubinov, M., Ypma, R. J. F., Watson, C. & Bullmore, E. T. Wiring cost and topological participation of the mouse brain connectome. Proc. Natl Acad. Sci. USA 112, 10032–10037 (2015).
Ferreira, F. R. M., Nogueira, M. I. & Defelipe, J. The influence of James and Darwin on Cajal and his research into the neuron theory and evolution of the nervous system. Front. Neuroanat. 8, 1 (2014).
Guimera, R., Mossa, S., Turtschi, A. & Amaral, L. A. N. The worldwide air transportation network: anomalous centrality, community structure, and cities' global roles. Proc. Natl Acad. Sci. USA 102, 7794–7799 (2005).
Guimerà, R., Sales-Pardo, M. & Amaral, L. A. N. Classes of complex networks defined by role-to-role connectivity profiles. Nat. Phys. 3, 63–69 (2007).
Power, J. D., Schlaggar, B. L., Lessov-Schlaggar, C. N. & Petersen, S. E. Evidence for hubs in human functional brain. Networks. 79, 798–813 (2013).
van den Heuvel, M. P. & Sporns, O. An anatomical substrate for integration among functional networks in human cortex. J. Neurosci. 33, 14489–14500 (2013).
Scholtens, L. H., Schmidt, R., de Reus, M. A. & van den Heuvel, M. P. Linking macroscale graph analytical organization to microscale neuroarchitectonics in the macaque connectome. J. Neurosci. 34, 12192–12205 (2014).
Cole, M. W. et al. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat. Neurosci. 16, 1348–1355 (2013).
Yeo, B. T. T. et al. Functional specialization and flexibility in human association cortex. Cereb. Cortex 25, 3654–3672 (2015).
Bassett, D. S., Yang, M., Wymbs, N. F. & Grafton, S. T. Learning-induced autonomy of sensorimotor systems. Nat. Neurosci. 18, 744–751 (2015).
Spadone, S. et al. Dynamic reorganization of human resting-state networks during visuospatial attention. Proc. Natl Acad. Sci. USA 112, 8112–8117 (2015).
Gratton, C., Laumann, T. O., Gordon, E. M., Adeyemo, B. & Petersen, S. E. Evidence for two independent factors that modify brain networks to meet task goals. Cell Rep. 17, 1276–1288 (2016).
Yamashita, M., Kawato, M. & Imamizu, H. Predicting learning plateau of working memory from whole-brain intrinsic network connectivity patterns. Sci. Rep. 5, 7622 (2015).
Baldassarre, A. et al. Individual variability in functional connectivity predicts performance of a perceptual task. Proc. Natl Acad. Sci. USA 109, 3516–3521 (2012).
Sala-Llonch, R. et al. Brain connectivity during resting state and subsequent working memory task predicts behavioural performance. Cortex 48, 1187–1196 (2012).
Sadaghiani, S., Poline, J.-B., Kleinschmidt, A. & D’Esposito, M. Ongoing dynamics in large-scale functional connectivity predict perception. Proc. Natl Acad. Sci. USA 112, 8463–8468 (2015).
Shine, J. M. et al. The dynamics of functional brain networks: integrated network states during cognitive task performance. Neuron 92, 544–554 (2016).
Lerman, C. et al. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry 71, 523–530 (2014).
Finn, E. S. et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 18, 1664–1671 (2015).
Van Essen, D. C. et al. The WU-Minn Human Connectome Project: an overview. NeuroImage 80, 62–79 (2013).
Power, J. D. et al. Functional network organization of the human brain. Neuron 72, 665–678 (2011).
Smith, S. M. et al. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat. Neurosci. 18, 1565–1567 (2015).
Bullmore, E. & Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 74, 47–14 (2012).
Cox, R. W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173 (1996).
Cole, M. W., Bassett, D. S., Power, J. D., Braver, T. S. & Petersen, S. E. Intrinsic and task-evoked network architectures of the human.Brain 83, 238–251 (2014).
Siegel, J. S., Mitra, A., Laumann, T. O. & Seitzman, B. A. Data quality influences observed links between functional connectivity and behavior. Cereb. Cortex 27, 4492–4502 (2016).
Gu, S., Satterthwaite, T. D. & Medaglia, J. D. Emergence of system roles in normative neurodevelopment. Proc. Natl Acad. Sci. USA 112, 13681–13686 (2015).
Zalesky, A., Fornito, A. & Bullmore, E. On the use of correlation as a measure of network connectivity. NeuroImage 60, 2096–2106 (2012).
Rosvall, M., Rosvall, M., Bergstrom, C. T. & Bergstrom, C. T. Maps of random walks on complex networks reveal community structure. Proc. Natl Acad. Sci. USA 105, 1118–1123 (2008).
Brandes, U., Delling, D. & Gaertler, M. On modularity clustering. IEEE Trans. Knowl. Data Eng. 20, 172–188 (2008).
Lancichinetti, A. & Fortunato, S. Community detection algorithms: a comparative analysis. Phys. Rev. E 80, 056117–11 (2009).
Berenstein, A. J., Piñero, J., Furlong, L. I. & Chernomoretz, A. Mining the modular structure of protein interaction networks. PLoS ONE 10, e0122477 (2015).
Clauset, A., Newman, M. & Moore, C. Finding community structure in very large networks. Phys. Rev. E 70, 066111 (2004).
Blondel, V. D., Guillaume, J.-L., Lambiotte, R. & Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, P10008 (2008).
WU-Minn HCP 1200 Subjects Data Release Reference Manual (Human Connectome Project, 2018); https://www.humanconnectome.org/storage/app/media/documentation/s1200/HCP_S1200_Release_Reference_Manual.pdf
Shen, X. et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat. Protoc. 12, 506–518 (2017).
Acknowledgements
This work was supported by National Institutes of Health (NIH) grant numbers NS79698 and the National Science Foundation Graduate Research Fellowship Program under grant number DGE 1106400 to M.A.B. and M.D. M.A.B. also acknowledges NIH T32 Ruth L. Kirschstein Institutional National Research Service Award (5T32MH106442-02). B.T.T.Y. was also supported by Singapore MOE Tier 2 (MOE2014-T2-2-016), NUS Strategic Research (DPRT/944/09/14), NUS SOM Aspiration Fund (R185000271720), Singapore NMRC (CBRG/0088/2015), NUS YIA and the Singapore National Research Foundation Fellowship (Class of 2017). D.S.B. also acknowledges support from the John D. and Catherine T. MacArthur Foundation, the Alfred P. Sloan Foundation, the Army Research Laboratory and the Army Research Office through contract numbers W911NF-10-2-0022 and W911NF-14-1-0679, the NIH (2-R01-DC-009209-11,1R01HD086888-01, R01-MH107235, R01-MH107703 and R21-M MH-106799), the Office of Naval Research and the National Science Foundation (BCS-1441502, PHY-1554488 and BCS-1631550). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
M.A.B. conceived the analyses. M.A.B., B.T.T.Y., D.S.B. and M.D. collaboratively designed the analyses. M.A.B. executed the analyses. M.A.B., B.T.T.Y., D.S.B. and M.D. collaboratively wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bertolero, M.A., Yeo, B.T.T., Bassett, D.S. et al. A mechanistic model of connector hubs, modularity and cognition. Nat Hum Behav 2, 765–777 (2018). https://doi.org/10.1038/s41562-018-0420-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-018-0420-6
This article is cited by
-
Investigating robust associations between functional connectivity based on graph theory and general intelligence
Scientific Reports (2024)
-
Connectome-based reservoir computing with the conn2res toolbox
Nature Communications (2024)
-
Disrupted longitudinal restoration of brain connectivity during weight normalization in severe anorexia nervosa
Translational Psychiatry (2023)
-
Sex differences of signal complexity at resting-state functional magnetic resonance imaging and their associations with the estrogen-signaling pathway in the brain
Cognitive Neurodynamics (2023)
-
The role of PFC networks in cognitive control and executive function
Neuropsychopharmacology (2022)