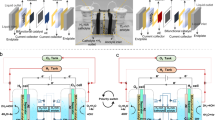
Abstract
Ion-solvating membranes (ISMs) are an alternative to proton-exchange and anion-exchange membranes for use in water electrolysers. ISMs do not have fixed ionic groups in their structure but instead gain their ionic conductivity through the uptake of liquid electrolyte. Although in principle they could offer improved stability over anion-exchange membranes due to the absence of easily degradable anion-exchange groups, stability gains have been modest. Here we report poly(oxindole biphenylene)-based ISMs with highly stable oxindole/KOH complex ion pairs for use in water electrolysers. These ISMs exhibit promising alkaline stability at 80 °C with a negligible conductivity decay over more than 15,000 h and, thus, allow durable alkaline electrolysis over 2,500 h, even at elevated temperatures and high operating voltages of 2.3 V. Moreover, they show ultralow gas permeation and, thus, low transient response times (<1 s). They allow the use of non-precious-metal catalysts (Ni and Ni/Fe) and can be operated over a broad temperature range (−35 to 120 °C).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper, Supplementary Information and Source Data files. Further data beyond the immediate results presented here are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Li, D. et al. Highly quaternized polystyrene ionomers for high performance anion exchange membrane water electrolysers. Nat. Energy 5, 378–385 (2020).
Abbasi, R. et al. A roadmap to low-cost hydrogen with hydroxide exchange membrane electrolyzers. Adv. Mater. 31, e1805876 (2019).
Schug, C. A. Operational characteristics of high-pressure, high-efficiency water-hydrogen-electrolysis. Int. J. Hydrogen Energy 23, 1113–1120 (1998).
Lohmann-Richters, F. P., Renz, S., Lehnert, W., Müller, M. & Carmo, M. Review—challenges and opportunities for increased current density in alkaline electrolysis by increasing the operating temperature. J. Electrochem. Soc. 168, 114501 (2021).
Lee, B., Lim, D., Lee, H. & Lim, H. Which water electrolysis technology is appropriate? Critical insights of potential water electrolysis for green ammonia production. Renew. Sustain. Energy Rev. 143, 110963 (2021).
Hu, X. et al. Piperidinium functionalized aryl ether-free polyaromatics as anion exchange membrane for water electrolysers: performance and durability. J. Membr. Sci. 621, 118964 (2021).
Jiao, K. et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 595, 361–369 (2021).
Olsson, J. S., Pham, T. H. & Jannasch, P. Poly(arylene piperidinium) hydroxide ion exchange membranes: synthesis, alkaline stability, and conductivity. Adv. Funct. Mater. 28, 1702758 (2018).
Li, D. et al. Durability of anion exchange membrane water electolyzers. Energy Environ. Sci. 14, 3393 (2021).
Holmes, T., Skalski, T. J. G., Adamski, M. & Holdcroft, S. Stability of hydrocarbon fuel cell membranes: reaction of hydroxyl radicals with sulfonated phenylated polyphenylenes. Chem. Mater. 31, 1441–1449 (2019).
Tang, H. et al. Fuel cells with an operational range of −20 °C to 200 °C enabled by phosphoric acid-doped intrinsically ultramicroporous membranes. Nat. Energy 7, 153–162 (2022).
Kraglund, M. R. et al. Ion-solvating membranes as a new approach towards high rate alkaline electrolyzers. Energy Environ. Sci. 12, 3313–3318 (2019).
Aili, D. et al. Towards a stable ion-solvating polymer electrolyte for advanced alkaline water electrolysis. J. Mater. Chem. A 5, 5055–5066 (2017).
Xing, B. & Savadogo, O. Hydrogen/oxygen polymer electrolyte membrane fuel cells (PEMFCs) based on alkaline-doped polybenzimidazole (PBI). Electrochem. Commun. 2, 697–702 (2000).
Hu, B. et al. A stable ion-solvating PBI electrolyte enabled by sterically bulky naphthalene for alkaline water electrolysis. J. Membr. Sci. 643, 120042 (2022).
Hu, X., Liu, M., Huang, Y., Liu, L. & Li, N. Sulfonate-functionalized polybenzimidazole as ion-solvating membrane toward high-performance alkaline water electrolysis. J. Membr. Sci. 663, 121005 (2022).
Grigoriev, S. A., Fateev, V. N., Bessarabov, D. G. & Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrogen Energy 45, 26036–26058 (2020).
Zhang, K. et al. Status and perspectives of key materials for PEM electrolyzer. Nano Res. Energy 1, e9120032 (2022).
Hernandez, M. C. G. et al. Novel, metal-free, superacid-catalyzed ‘click’ reactions of isatins with linear, nonactivated, multiring aromatic hydrocarbons. Macromolecules 43, 6968–6979 (2010).
Hossain, I., Husna, A., Jeong, I. & Kim, T.-H. Biphenyl(isatin-co-trifluoroacetophenone)-based copolymers synthesized using the Friedel–Crafts reaction as mechanically robust membranes for efficient CO2 separation. ACS Appl. Polym. Mater. 4, 3779–3790 (2022).
Zhang, S., Zhu, X. & Jin, C. Development of a high-performance anion exchange membrane using poly(isatin biphenylene) with flexible heterocyclic quaternary ammonium cations for alkaline fuel cells. J. Mater. Chem. A 7, 6883–6893 (2019).
Pulido, B., Chisca, S. & Nunes, S. P. Solvent and thermal resistant ultrafiltration membranes from alkyne-functionalized high-performance polymers. J. Membr. Sci. 564, 361–371 (2018).
Lin, B. et al. Alkaline stable C2-substituted imidazolium-based anion-exchange membranes. Chem. Mater. 25, 1858–1867 (2013).
Klumpp, D. A., Yeung, K. Y., Surya Prakash, G. K. & Olah, G. A. Preparation of 3,3-diaryloxindoles by superacid-induced condensations of isatins and aromatics with a combinatorial approach. J. Org. Chem. 63, 4 (1998).
Cruz, A. R. et al. Precision synthesis of narrow polydispersity, ultrahigh molecular weight linear aromatic polymers by A2 + B2 nonstoichiometric step-selective polymerization. Macromolecules 45, 6774–6780 (2012).
Bruce, J. M. & Sutcliffe, F. K. Heterocyclic compounds of nitrogen. Part I. The alkylation and acylation of 3-phenyloxindole, and the preparation of some derivatives of 2-hydroxy-3-phenylindole, J. Chem. Soc. 1957, 4789–4798 (1957).
Bordwell, F. G. & Fried, H. E. Heterocyclic aromatic anions with 4n + 2 π-electrons. J. Org. Chem. 56, 4218–4224 (1991).
Wang, J. W., Jiang, L., Huang, H. H., Han, Z. & Ouyang, G. Rapid electron transfer via dynamic coordinative interaction boosts quantum efficiency for photocatalytic CO2 reduction. Nat. Commun. 12, 4276 (2021).
Zeng, L., Zhao, T. S., An, L., Zhao, G. & Yan, X. H. Physicochemical properties of alkaline doped polybenzimidazole membranes for anion exchange membrane fuel cells. J. Membr. Sci. 493, 340–348 (2015).
Aili, D. et al. Understanding ternary poly(potassium benzimidazolide)-based polymer electrolytes. Polymer 84, 304–310 (2016).
Chang, Z. et al. Chemical oxidative degradation of polybenzimidazole in simulated environment of fuel cells. Polym. Degrad. Stab. 94, 1206–1212 (2009).
Wang, J. et al. Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells. Nat. Energy 4, 392–398 (2019).
Kraglund, M. R. et al. Zero-gap alkaline water electrolysis using ion-solvating polymer electrolyte membranes at reduced KOH concentrations. J. Electrochem. Soc. 163, F3125–F3131 (2016).
Choe, Y.-K. et al. Alkaline stability of benzyl trimethyl ammonium functionalized polyaromatics: a computational and experimental study. Chem. Mater. 26, 5675–5682 (2014).
Vengatesan, S., Santhi, S., Jeevanantham, S. & Sozhan, G. Quaternized poly (styrene-co-vinylbenzyl chloride) anion exchange membranes for alkaline water electrolysers. J. Power Sources 284, 361–368 (2015).
Liu, Z. et al. The effect of membrane on an alkaline water electrolyzer. Int. J. Hydrogen Energy 42, 29661–29665 (2017).
Chu, X., Shi, Y., Liu, L., Huang, Y. & Li, N. Piperidinium-functionalized anion exchange membranes and their application in alkaline fuel cells and water electrolysis. J. Mater. Chem. A 7, 7717–7727 (2019).
Li, D. et al. Phenyl oxidation impacts the durability of alkaline membrane water electrolyzer. ACS Appl. Mater. Interfaces 11, 9696–9701 (2019).
Li, H. et al. Poly(vinyl benzyl methylpyrrolidinium) hydroxide derived anion exchange membranes for water electrolysis. J. Mater. Chem. A 7, 17914–17922 (2019).
Su, X. et al. Novel piperidinium functionalized anionic membrane for alkaline polymer electrolysis with excellent electrochemical properties. J. Membr. Sci. 581, 283–292 (2019).
Cha, M. S. et al. Poly(carbazole)-based anion-conducting materials with high performance and durability for energy conversion devices. Energy Environ. Sci. 13, 3633–3645 (2020).
Lee, H. I. et al. Advanced Zirfon-type porous separator for a high-rate alkaline electrolyser operating in a dynamic mode. J. Membr. Sci. 616, 118541 (2020).
Park, H. J., Lee, S. Y., Lee, T. K., Kim, H.-J. & Lee, Y. M. N3-butyl imidazolium-based anion exchange membranes blended with poly(vinyl alcohol) for alkaline water electrolysis. J. Membr. Sci. 611, 118355 (2020).
Park, Y. S. et al. Superior performance of anion exchange membrane water electrolyzer: ensemble of producing oxygen vacancies and controlling mass transfer resistance. Appl. Catal. B 278, 119276 (2020).
Yan, X. et al. Twisted ether-free polymer based alkaline membrane for high-performance water electrolysis. J. Power Sources 480, 228805 (2020).
Chen, N. et al. High-performance anion exchange membrane water electrolyzers with a current density of 7.68 A cm−2 and durability of 1000 h. Energy Environ. Sci. 14, 6338–6348 (2021).
Xu, Z. et al. Anisotropic anion exchange membranes with extremely high water uptake for water electrolysis and fuel cell. J. Mater. Chem. A 9, 23485 (2021).
Liu, M., Hu, X., Hu, B., Liu, L. & Li, N. Soluble poly(aryl piperidinium) with extended aromatic segments as anion exchange membranes for alkaline fuel cells and water electrolysis. J. Membr. Sci. 642, 119966 (2022).
Diaz, L. A. et al. Alkali-doped polyvinyl alcohol – polybenzimidazole membranes for alkaline water electrolysis. J. Membr. Sci. 535, 45–55 (2017).
Konovalova, A. et al. Blend membranes of polybenzimidazole and an anion exchange ionomer (FAA3) for alkaline water electrolysis: improved alkaline stability and conductivity. J. Membr. Sci. 564, 653–662 (2018).
Acknowledgements
We thank X. Zhang from Nanjing University of Science and Technology for the simulations and M. Dou at the Institute of Coal Chemistry of the Chinese Academy of Sciences for help with the NMR. We also thank Shiyanjia Lab (www.shiyanjia.com) for support during the DFT test. Financial support for this work was provided by the National Natural Science Foundation of China (Grant Nos. 21835005 and 22105217), the STS Project of the Chinese Academy of Sciences (Grant No. KFJ-STS-QYZD-2021-02-003) and the Natural Science Foundation of Shanxi Province (Grant No. 20210302124433).
Author information
Authors and Affiliations
Contributions
N.L. initiated this collaborative project. K.G. helped with data collection and the formal analysis. X.H. synthesized the polymeric materials, designed the experiments and wrote the original draft of the manuscript. C.N. and J.Y. set up the testing system at high operating pressure and high temperature and collected the data. K.G., B.H., M.L., H.T. and S.K. characterized the electrolyser tests and reviewed the draft of the manuscript. Y.H., N.L. and K.G. contributed to the writing of the manuscript. K.G. and N.L. supervised and guided the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Patric Jannasch, Jens Oluf Jensen and Yu Seung Kim for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3 show the alkaline stability of a small molecule. Supplementary Fig. 4 shows the dynamic thermomechanical analysis of POBP. Supplementary Figs. 5–14 show the swelling behaviour, XRD, structure and hydrophily of all the POBPs. Supplementary Figs. 15 and 16 show the thermal stability of POBP. Supplementary Figs. 17–19 show the alkaline and oxidative stability of POBP. Supplementary Figs. 20–28 show the AWE device performance of POBP. Supplementary Figs. 29–35 show the in situ stability of POBP under different conditionals. Supplementary Table 1 lists the solubility of the POBP and Supplementary Table 2 lists the polymerization conditions of the POBP.
Supplementary Data 1
Source Data for Supplementary Figs. 1–35.
Source data
Source Data for Fig. 1
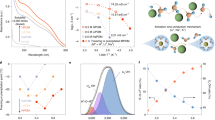
DFT calculation and alkaline stability test of model compounds.
Source Data for Fig. 2
Preparation, composition, mechanical properties and gas permeability characterization of POBP-ISMs.
Source Data for Fig. 3
Ion conductivity, alkaline durability, oxidation stability of POBP and 1H NMR spectra of pristine and aged POBP after alkaline during test and Fenton test.
Source Data for Fig. 4
Polarization curves of POBP-ISM AWE under different conditions and area resistance (AR).
Source Data for Fig. 5
In situ durability of electrolyser with POBP-ISMs at 80 °C and comparison of in situ durability and cell voltage of present POBP-ISM and current ISMs and AEMs.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, X., Hu, B., Niu, C. et al. An operationally broadened alkaline water electrolyser enabled by highly stable poly(oxindole biphenylene) ion-solvating membranes. Nat Energy 9, 401–410 (2024). https://doi.org/10.1038/s41560-023-01447-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01447-w