Abstract
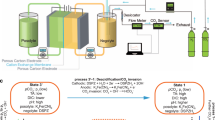
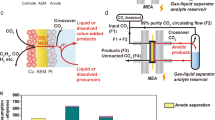
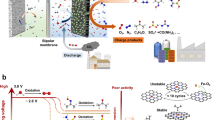
Carbon dioxide capture technologies will be important for counteracting difficult-to-abate greenhouse gas emissions if humanity is to limit global warming to acceptable levels. Electrochemically mediated CO2 capture has emerged as a promising alternative to conventional amine scrubbing, offering a potentially cost effective, environmentally friendly and energy efficient approach. Here we report an electrochemical cell for CO2 capture based on pH swing cycles driven through proton-coupled electron transfer of a developed phenazine derivative, 2,2′-(phenazine-1,8-diyl)bis(ethane-1-sulfonate) (1,8-ESP), with high aqueous solubility (>1.35 M) over pH range 0.00–14.90. The system operates with a high capture capacity of 0.86–1.41 mol l−1, a low energetic cost of 36–55 kJ mol−1 and an extremely low capacity fade rate of <0.01% per day, depending on organic concentration. The system charge–discharge cycle provides an electrical energy storage function that could be run only for storage when called for by electricity market conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in the published article and its Supplementary Information file. Source data are provided with this paper.
References
National Centers for Environmental Information State of the Climate: Global Climate Report for 2021 (NOAA, 2021).
Negative Emissions Technologies and Reliable Sequestration: A Research Agenda (National Academies Press, 2019).
Raupach, M. R. et al. Global and regional drivers of accelerating CO2 emissions. Proc. Natl Acad. Sci. USA 104, 10288–10293 (2007).
Pacala, S. & Socolow, R. Stabilization wedges: solving the climate problem for the next 50 years with current technologies. Science 305, 968–972 (2004).
Wang, Y., Zhao, L., Otto, A., Robinius, M. & Stolten, D. A review of post-combustion CO2 capture technologies from coal-fired power plants. Energy Proc. 114, 650–665 (2017).
About CCUS (IEA, 2021); https://www.iea.org/reports/about-ccus
Global Status of CCS 2022 (Global CCS Institute, 2022); https://www.globalccsinstitute.com/resources/global-status-of-ccs-2022/
Keith, D. W. Why capture CO2 from the atmosphere? Science 325, 1654–1655 (2009).
Shi, X. et al. Sorbents for the direct capture of CO2 from ambient air. Angew. Chem. Int. Ed. 59, 6984–7006 (2020).
IPCC Special Report on Global Warming of 1.5 °C (eds Masson-Delmotte, V. et al.) (WMO, 2018).
Rochelle, G. T. Amine scrubbing for CO2 capture. Science 325, 1652–1654 (2009).
Luis, P. Use of monoethanolamine (MEA) for CO2 capture in a global scenario: consequences and alternatives. Desalination 380, 93–99 (2016).
Keith, D. W., Holmes, G., St. Angelo, D. & Heidel, K. A process for capturing CO2 from the atmosphere. Joule 2, 1573–1594 (2018).
Rinberg, A., Bergman, A. M., Schrag, D. P. & Aziz, M. J. Alkalinity concentration swing for direct air capture of carbon dioxide. ChemSusChem 14, 4439–4453 (2021).
Sanz-Pérez, E. S., Murdock, C. R., Didas, S. A. & Jones, C. W. Direct capture of CO2 from ambient air. Chem. Rev. 116, 11840–11876 (2016).
McQueen, N. et al. A review of direct air capture (DAC): scaling up commercial technologies and innovating for the future. Prog. Energy 3, 032001 (2021).
Kang, J. S., Kim, S. & Hatton, T. A. Redox-responsive sorbents and mediators for electrochemically based CO2 capture. Curr. Opin. Green Sustain. Chem. 31, 100504 (2021).
Muroyama, A. P., Pătru, A. & Gubler, L. Review—CO2 separation and transport via electrochemical methods. J. Electrochem. Soc. 167, 133504 (2020).
de Lannoy, C.-F. et al. Indirect ocean capture of atmospheric CO2: part I. Prototype of a negative emissions technology. Int. J. Greenhouse Gas Control 70, 243–253 (2018).
Rheinhardt, J. H., Singh, P., Tarakeshwar, P. & Buttry, D. A. Electrochemical capture and release of carbon dioxide. ACS Energy Lett. 2, 454–461 (2017).
Sharifian, R., Wagterveld, R. M., Digdaya, I. A., Xiang, C. & Vermaas, D. A. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 14, 781–814 (2021).
Jin, S., Wu, M., Gordon, R. G., Aziz, M. J. & Kwabi, D. G. pH swing cycle for CO2 capture electrochemically driven through proton-coupled electron transfer. Energy Environ. Sci. 13, 3706–3722 (2020).
Jin, S., Wu, M., Jing, Y., Gordon, R. G. & Aziz, M. J. Low energy carbon capture via electrochemically induced pH swing with electrochemical rebalancing. Nat. Commun. 13, 2140 (2022).
Huang, C. et al. CO2 capture from flue gas using an electrochemically reversible hydroquinone/quinone solution. Energy Fuels 33, 3380–3389 (2019).
Xie, H. et al. Low-energy-consumption electrochemical CO2 capture driven by biomimetic phenazine derivatives redox medium. Appl. Energy 259, 114119 (2020).
Xie, H. et al. Low-energy electrochemical carbon dioxide capture based on a biological redox proton carrier. Cell Rep. Phys. Sci. 1, 100046 (2020).
Kwabi, D. G., Ji, Y. L. & Aziz, M. J. Electrolyte lifetime in aqueous organic redox flow batteries: a critical review. Chem. Rev. 120, 6467–6489 (2020).
Voskian, S. & Hatton, T. A. Faradaic electro-swing reactive adsorption for CO2 capture. Energy Environ. Sci. 12, 3530–3547 (2019).
Rahimi, M. et al. An electrochemically mediated amine regeneration process with a mixed absorbent for postcombustion CO2 capture. Environ. Sci. Technol. 54, 8999–9007 (2020).
Salles, M. B. C., Gadotti, T. N., Aziz, M. J. & Hogan, W. W. Potential revenue and breakeven of energy storage systems in PJM energy markets. Environ. Sci. Pollut. Res. 28, 12357–12368 (2021).
Kwabi, D. G. et al. Alkaline quinone flow battery with long lifetime at pH 12. Joule 2, 1894–1906 (2018).
Jin, S. et al. Near neutral pH redox flow battery with low permeability and long-lifetime phosphonated viologen active species. Adv. Energy Mater. 10, 2000100 (2020).
Tiwari, S. P. et al. Foaming dependence on the interface affinities of surfactant-like molecules. Ind. Eng. Chem. Res. 58, 19877–19889 (2019).
Diederichsen, K. M., Liu, Y., Ozbek, N., Seo, H. & Hatton, T. A. Toward solvent-free continuous-flow electrochemically mediated carbon capture with high-concentration liquid quinone chemistry. Joule 6, 221–239 (2022).
Goto, K., Kodama, S., Higashii, T. & Kitamura, H. Evaluation of amine-based solvent for post-combustion capture of carbon dioxide. J. Chem. Eng. Jpn 47, 663–665 (2014).
House, K. Z., Harvey, C. F., Aziz, M. J. & Schrag, D. P. The energy penalty of post-combustion CO2 capture & storage and its implications for retrofitting the U.S. installed base. Energy Environ. Sci. 2, 193–205 (2009).
Hitchcock, L. B. Rate of absorption of carbon dioxide effect of concentration and viscosity of caustic solutions. Ind. Eng. Chem. 26, 1158–1167 (1934).
Hu, G. et al. Carbon dioxide absorption into promoted potassium carbonate solutions: a review. Int. J. Greenhouse Gas Control 53, 28–40 (2016).
Pinsent, B. R. W., Pearson, L. & Roughton, F. J. W. The kinetics of combination of carbon dioxide with hydroxide ions. Trans. Faraday Soc. 52, 1512–1520 (1956).
Ji, Y. et al. A phosphonate-functionalized quinone redox flow battery at near-neutral pH with record capacity retention rate. Adv. Energy Mater. 9, 1900039 (2019).
Pang, S., Wang, X., Wang, P. & Ji, Y. Biomimetic amino acid functionalized phenazine flow batteries with long lifetime at near-neutral pH. Angew. Chem. Int. Ed. 60, 5289–5298 (2021).
Xu, J., Pang, S., Wang, X., Wang, P. & Ji, Y. Ultrastable aqueous phenazine flow batteries with high capacity operated at elevated temperatures. Joule 5, 2437–2449 (2021).
Liu, Y., Ye, H.-Z., Diederichsen, K. M., Van Voorhis, T. & Hatton, T. A. Electrochemically mediated carbon dioxide separation with quinone chemistry in salt-concentrated aqueous media. Nat. Commun. 11, 2278 (2020).
Dell’Amico, L., Bonchio, M. & Companyó, X. Recent Advances in Electrochemical Carboxylation of Organic Compounds for CO2 Valorization. In CO2 as a Building Block in Organic Synthesis 225–252 (2020). (ed Shoubhik Das) (WILEY-VCH, 2020)
Li, X., Zhao, X. H., Liu, Y. Y., Hatton, T. A. & Liu, Y. Y. Redox-tunable Lewis bases for electrochemical carbon dioxide capture. Nat. Energy 7, 1065–1075 (2022).
Xie, W. et al. Fundamental salt and water transport properties in directly copolymerized disulfonated poly(arylene ether sulfone) random copolymers. Polymer 52, 2032–2043 (2011).
Beh, E. S. et al. A neutral pH aqueous organic–organometallic redox flow battery with extremely high capacity retention. ACS Energy Lett. 2, 639–644 (2017).
Treimer, S., Tang, A. & Johnson, D. C. A consideration of the application of Koutecký–Levich plots in the diagnoses of charge-transfer mechanisms at rotated disk electrodes. Electroanalysis 14, 165–171 (2002).
Sakai, M. & Ohnaka, N. Kinetics of the second charge transfer step in an ee mechanism by rotating ring‐disk electrode voltammetry. J. Electrochem. Soc. 137, 576–579 (1990).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Clark, T., Chandrasekhar, J., Spitznagel, G. W. & Schleyer, P. V. R. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3–21 + G basis set for first-row elements, Li–F. J. Comput. Chem. 4, 294–301 (1983).
Francl, M. M. et al. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 77, 3654–3665 (1982).
van der Bondi, A. Waals volumes and radii. J. Phys. Chem. 68, 441–451 (1964).
Ho, J., Klamt, A. & Coote, M. L. Comment on the correct use of continuum solvent models. J. Phys. Chem. A 114, 13442–13444 (2010).
Savitzky, A. & Golay, M. J. E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 36, 1627–1639 (1964).
Acknowledgements
Financial support received from the National Natural Science Foundation of China (22005249, 22101064) and Zhejiang Leading Innovative and Entrepreneur Team Introduction Program (2020R01004) is gratefully acknowledged. Research at Harvard University was supported by the Harvard University Climate Change Solutions Fund. We thank the Instrumentation and Service Center for Molecular Sciences (ISCMS) and HPC Center at Westlake University for the facility support and technical assistance. We thank D.P. Schrag, T. George, A. Rinberg, Y. Jing and A. Bergman for helpful discussions. We thank X. Lu, X. Shi, Y. Chen and K. Wang for data collection and helpful discussions.
Author information
Authors and Affiliations
Contributions
P.W., Y.J., R.G.G. and M.J.A. formulated and supervised the project. S.P. and F.Y. synthesized the compounds. S.J., M.A. and D.X. performed the CO2 capture tests. S.P. performed the cell cycling tests. L.L. performed theoretical analysis. S.J., P.W., Y.J. and M.J.A. wrote the paper, and all authors contributed to revising the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Yayuan Liu, Shrihari Sankarasubramanian and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–39, Tables 1–5 and Notes 1 and 2.
Source data
Source Data Fig. 3
Source data for the CO2 concentrating cycle using a 1,8-ESP-based flow cell.
Source Data Fig. 4
Source data for CO2 separation performance at different 1,8-ESP concentrations and current densities.
Source Data Fig. 5
Source data for the cycling performance of 0.1 M 1,8-ESP full cell.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pang, S., Jin, S., Yang, F. et al. A phenazine-based high-capacity and high-stability electrochemical CO2 capture cell with coupled electricity storage. Nat Energy 8, 1126–1136 (2023). https://doi.org/10.1038/s41560-023-01347-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01347-z