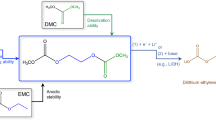
Abstract
Conventional carbonate-based electrolytes with high corrosion towards Li metal result in massive dendrite growth and limited cycling life, particularly true for practical Li-metal batteries with high cathode loading (>3.5 mAh cm−2). Herein we design an asymmetric Li salt, lithium 1,1,1-trifluoro-N-[2-[2-(2-methoxyethoxy)ethoxy)]ethyl] methanesulfonamide (LiFEA) that possesses a pseudo-crown ether-like, folded molecular geometry. It enables carbonate electrolytes with a large apparent donor number and Li+ transference number and drives a self-cleaning mechanism for solid–electrolyte interphases, enhancing compatibility with Li-metal anodes even at high current densities. LiFEA-based carbonate electrolytes notably improved fast-cycling performances of Li | |NCM811 cells. Pouch cells of 310 Wh kg−1 achieved ~410 W kg−1 power density at the discharging current density of 6.59 mA cm−2. Under fast-cycling conditions (charging: 1.46 mA cm−2, discharging: 3.66 mA cm−2), pouch cells maintained 81% capacity after 100 cycles. Our work provides insights into the interplay between the molecular structure of Li salts, their physicochemical properties and electrochemical performances.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The datasets analysed and generated during the current study are included in the paper and its Supplementary Information file. Source data are provided with this paper.
References
Niu, C. et al. Self-smoothing anode for achieving high-energy lithium metal batteries under realistic conditions. Nat. Nanotechnol. 14, 594–601 (2019).
Holoubek, J. et al. Tailoring electrolyte solvation for Li metal batteries cycled at ultra-low temperature. Nat. Energy 6, 303–313 (2021).
Zhang, Z. et al. Capturing the swelling of solid-electrolyte interphase in lithium metal batteries. Science 375, 66–70 (2022).
Xiao, J. et al. Understanding and applying Coulombic efficiency in lithium metal batteries. Nat. Energy 5, 561–568 (2020).
Kim, M. S. et al. Suspension electrolyte with modified Li+ solvation environment for lithium metal batteries. Nat. Mater. 21, 445–454 (2022).
Cheng, X.-B., Zhang, R., Zhao, C.-Z. & Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: a review. Chem. Rev. 117, 10403–10473 (2017).
Liu, Y. et al. Self-assembled monolayers direct a LiF-rich interphase toward long-life lithium metal batteries. Science 375, 739–745 (2022).
Fan, X. et al. Non-flammable electrolyte enables Li-metal batteries with aggressive cathode chemistries. Nat. Nanotechnol. 13, 715–722 (2018).
Zhang, X.-Q. et al. Regulating anions in the solvation sheath of lithium ions for stable lithium metal batteries. ACS Energy Lett. 4, 411–416 (2019).
Zheng, J. et al. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2, 17012 (2017).
Zhou, P. et al. Rationally designed fluorinated amide additive enables the stable operation of lithium metal batteries by regulating the interfacial chemistry. Nano Lett. 22, 5936–5943 (2022).
Wang, H. et al. Dual-solvent Li-ion solvation enables high-performance Li-metal batteries. Adv. Mater. 33, 2008619 (2021).
Su, C. C. et al. Solvation rule for solid-electrolyte interphase enabler in lithium-metal batteries. Angew. Chem. Int. Ed. 59, 18229–18233 (2020).
Zhang, W. et al. Engineering a passivating electric double layer for high performance lithium metal batteries. Nat. Commun. 13, 2029 (2022).
Dong, H. et al. A thermoresponsive composite separator loaded with paraffin@SiO2 microparticles for safe and stable lithium batteries. J. Energy Chem. 62, 423–430 (2021).
Rustomji Cyrus, S. et al. Liquefied gas electrolytes for electrochemical energy storage devices. Science 356, eaal4263 (2017).
Yamada, Y. et al. Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy 4, 269–280 (2019).
Ren, X. et al. Enabling high-voltage lithium-metal batteries under practical conditions. Joule 3, 1662–1676 (2019).
Fan, X. et al. Highly fluorinated interphases enable high-voltage Li-metal batteries. Chem 4, 174–185 (2018).
Qiao, L. et al. Stable non-corrosive sulfonimide salt for 4-V-class lithium metal batteries. Nat. Mater. 21, 455–462 (2022).
Amanchukwu, C. V. et al. A new class of ionically conducting fluorinated ether electrolytes with high electrochemical stability. J. Am. Chem. Soc. 142, 7393–7403 (2020).
Xue, W. et al. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. Nat. Energy 6, 495–505 (2021).
Diederichsen, K. M., McShane, E. J. & McCloskey, B. D. Promising routes to a high Li+ transference number electrolyte for lithium ion batteries. ACS Energy Lett. 2, 2563–2575 (2017).
Liu, Y., Zhu, Y. & Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 4, 540–550 (2019).
Kakiuchi, F. et al. Ruthenium-catalyzed functionalization of aryl carbon−oxygen bonds in aromatic ethers with organoboron compounds. J. Am. Chem. Soc. 126, 2706–2707 (2004).
Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4418 (2004).
Nakatsuji, Y. et al. Molecular design of the electron-donating sidearm of lariat ethers: effective coordination of the quinoline moiety in complexation toward alkali-metal cations. J. Am. Chem. Soc. 110, 531–538 (1988).
Baxter, N. J. & Williamson, M. P. Temperature dependence of 1H chemical shifts in proteins. J. Biomol. NMR 9, 359–369 (1997).
Gutmann, V. & Wychera, E. Coordination reactions in non aqueous solutions—the role of the donor strength. Inorg. Nucl. Chem. Lett. 2, 257–260 (1966).
Schmeisser, M. et al. Gutmann donor and acceptor numbers for ionic liquids. Chem. Eur. J. 18, 10969–10982 (2012).
Johnson, L. et al. The role of LiO2 solubility in O2 reduction in aprotic solvents and its consequences for Li-O2 batteries. Nat. Chem. 6, 1091–1099 (2014).
Baek, M., Shin, H., Char, K. & Choi, J. W. New high donor electrolyte for lithium–sulfur batteries. Adv. Mater. 32, 2005022 (2020).
Kwon, H., Baek, J. & Kim, H.-T. Building lithium metal batteries under lean electrolyte conditions: challenges and progress. Energy Storage Mater. 55, 708–726 (2023).
Li, S. et al. A robust all-organic protective layer towards ultrahigh-rate and large-capacity Li metal anodes. Nat. Nanotechnol. 17, 613–621 (2022).
Ji, Y. et al. From bulk to interface: electrochemical phenomena and mechanism studies in batteries via electrochemical quartz crystal microbalance. Chem. Soc. Rev. 50, 10743–10763 (2021).
Jin, C. et al. Rejuvenating dead lithium supply in lithium metal anodes by iodine redox. Nat. Energy 6, 378–387 (2021).
Choudhury, S. et al. Designing solid-liquid interphases for sodium batteries. Nat. Commun. 8, 898 (2017).
Biswal, P. et al. A reaction-dissolution strategy for designing solid electrolyte interphases with stable energetics for lithium metal anodes. Cell Rep. Phys. Sci. 3, 100948 (2022).
Liu, S. et al. An inorganic-rich solid electrolyte interphase for advanced lithium-metal batteries in carbonate electrolytes. Angew. Chem. Int. Ed. 60, 3661–3671 (2021).
Dillon, R. E. A. & Shriver, D. F. Thermal and complex impedance analysis of amorphous and crystalline lithium salt mixtures. Solid State Ionics 140, 375–380 (2001).
Acknowledgements
This work was supported by the National Nature Science Fund of China (grant number 22071133, K. L.), Beijing Natural Science Foundation (grant number Z220020, K.L.), Recruitment Program of Guangdong (grant number 2016ZT06C322, X.K.), China Postdoctoral Science Foundation (grant number 2021M701872, Y.X.) and TCL Science and Technology Innovation Fund (X.K.). We thank C. Guo and Z. Li from Analysis Center, Tsinghua University for analysing the TOF-SIMs data, Y. Li from State Key Laboratory of Space Power-Sources Technology, Shanghai Institute of Space Power Sources for helpful discussion and C. Cui from Chemistry and Chemical Engineering, Hunan University for discussing electrochemical measurements. We thank J. Fang (Tsinghua University) and Biolin Scientific AB for QCM experiments and data analysis.
Author information
Authors and Affiliations
Contributions
Y.X. and K.L. conceived the idea and designed the experiments. Y.X., P.Z., X.K., J.T., W.Z., S.Y., W.-h.H., H.-Y.Z., Z.X. and L.W. carried out the experiments and measurements. H.D., X.C., P.W. and B.W. helped with discussion. Y.X., X.K. and K.L. analysed the data and prepared the manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
For K. Liu and Y. Xia, this work has been filed as a China invention patent, patent number CN113871718B. The other authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Hee-Tak Kim, Yuki Yamada and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–57, Notes 1–7 and Tables 1–7.
Source data
Source Data Fig. 3
Donor number data.
Source Data Fig. 5
Cycling data for pouch cells.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, Y., Zhou, P., Kong, X. et al. Designing an asymmetric ether-like lithium salt to enable fast-cycling high-energy lithium metal batteries. Nat Energy 8, 934–945 (2023). https://doi.org/10.1038/s41560-023-01282-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01282-z
This article is cited by
-
Hybridizing carbonate and ether at molecular scales for high-energy and high-safety lithium metal batteries
Nature Communications (2024)
-
Enhancing the electrochemical performance of Na metal anodes via local eutectic melting in porous Al-Cu alloy hosts
Nano Research (2024)
-
Enabling an Inorganic-Rich Interface via Cationic Surfactant for High-Performance Lithium Metal Batteries
Nano-Micro Letters (2024)
-
Leaching organics from the interphase
Nature Energy (2023)
-
Design of new chemicals for advanced electrolytes
Science China Chemistry (2023)