Abstract
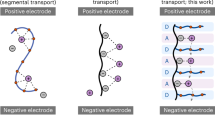
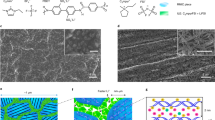
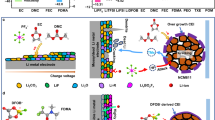
Electrically conductive polymers have found increasing applications in energy conversion and storage devices. In the conventional design of conductive polymers, organic functionalities are introduced via bottom-up synthetic approaches to enhance specific properties by modification of the individual polymers. Unfortunately, the addition of functional groups leads to conflicting effects, limiting their scaled synthesis and broad applications. Here we show a conductive polymer with simple primary building blocks that can be thermally processed to develop hierarchically ordered structures (HOS) with well-defined nanocrystalline morphologies. Our approach to constructing permanent HOS in conductive polymers leads to substantial enhancement of charge transport properties and mechanical robustness, which are critical for practical lithium-ion batteries. Finally, we demonstrate that conductive polymers with HOS enable exceptional cycling performance of full cells with high-loading micron-size SiOx-based anodes, delivering areal capacities of more than 3.0 mAh cm−2 over 300 cycles and average Coulombic efficiency of >99.95%.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All datasets analysed and generated during the current study are available in the paper or Supplementary Information. Source data are provided with this paper.
References
Akamatu, H., Inokuchi, H. & Matsunaga, Y. Electrical conductivity of the perylene–bromine complex. Nature 173, 168–169 (1954).
Jerome, D., Mazaud, A., Ribault, M. & Bechgaard, K. Superconductivity in a synthetic organic conductor (TMTSF)2PF6. J. Phys. 41, L95–L98 (1980).
Macdiarmid, A. G., Mammone, R. J., Kaner, R. B. & Porter, S. J. The concept of ‘doping’ of conducting polymers: the role of reduction potentials. Phil. Trans. R. Soc. A 314, 3–15 (1985).
Heeger, A. J. Semiconducting and metallic polymers: the fourth generation of polymeric materials (Nobel lecture). Angew. Chem. Int. Ed. 40, 2591–2611 (2001).
Paulsen, B. D., Tybrandt, K., Stavrinidou, E. & Rivnay, J. Organic mixed ionic–electronic conductors. Nat. Mater. 19, 13–26 (2020).
Guo, X. & Facchetti, A. The journey of conducting polymers from discovery to application. Nat. Mater. 19, 922–928 (2020).
Ho, P. K. et al. Molecular-scale interface engineering for polymer light-emitting diodes. Nature 404, 481–484 (2000).
Kaur, G., Adhikari, R., Cass, P., Bown, M. & Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 5, 37553–37567 (2015).
Brabec, C. J., Sariciftci, N. S. & Hummelen, J. C. Plastic solar cells. Adv. Funct. Mater. 11, 15–26 (2001).
Patil, A. O., Ikenoue, Y., Wudl, F. & Heeger, A. J. Water soluble conducting polymers. J. Am. Chem. Soc. 109, 1858–1859 (1987).
Pei, J., Yu, W. L., Huang, W. & Heeger, A. J. A novel series of efficient thiophene-based light-emitting conjugated polymers and application in polymer light-emitting diodes. Macromolecules 33, 2462–2471 (2000).
Ruhe, J., Ezquerra, T. A. & Wegner, G. New conducting polymers from 3-alkylpyrroles. Synth. Met. 28, C177–C181 (1989).
Liu, G. et al. Polymers with tailored electronic structure for high capacity lithium battery electrodes. Adv. Mater. 23, 4679–4683 (2011).
Huang, Y. H. & Goodenough, J. B. High-rate LiFePO4 lithium rechargeable battery promoted by electrochemically active polymers. Chem. Mater. 20, 7237–7241 (2008).
Xu, J. et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 355, 59–64 (2017).
Xie, L.-H., Yin, C.-R., Lai, W.-Y., Fan, Q.-L. & Huang, W. Polyfluorene-based semiconductors combined with various periodic table elements for organic electronics. Prog. Polym. Sci. 37, 1192–1264 (2012).
Smits, E. C. et al. Bottom-up organic integrated circuits. Nature 455, 956–959 (2008).
Fratini, S., Nikolka, M., Salleo, A., Schweicher, G. & Sirringhaus, H. Charge transport in high-mobility conjugated polymers and molecular semiconductors. Nat. Mater. 19, 491–502 (2020).
Noriega, R. et al. A general relationship between disorder, aggregation and charge transport in conjugated polymers. Nat. Mater. 12, 1038–1044 (2013).
Ezquerra, T. A., Ruhe, J. & Wegner, G. Hopping conduction in 3,4-cycloalkylpolypyrrole perchlorates: a model study of conductivity in polymers. Chem. Phys. Lett. 144, 194–198 (1988).
Wu, M. Y. et al. Toward an ideal polymer binder design for high-capacity battery anodes. J. Am. Chem. Soc. 135, 12048–12056 (2013).
Maria, I. P. et al. The effect of alkyl spacers on the mixed ionic-electronic conduction properties of N-type polymers. Adv. Funct. Mater. 31, 2008718 (2021).
Stavrinidou, E. et al. Direct measurement of ion mobility in a conducting polymer. Adv. Mater. 25, 4488–4493 (2013).
Baran, M. J. et al. Diversity-oriented synthesis of polymer membranes with ion solvation cages. Nature 592, 225–231 (2021).
Lopez, J., Mackanic, D. G., Cui, Y. & Bao, Z. Designing polymers for advanced battery chemistries. Nat. Rev. Mater. 4, 312–330 (2019).
Blundell, T., Sibanda, B., Sternberg, M. & Thornton, J. Knowledge-based prediction of protein structures and the design of novel molecules. Nature 326, 347–352 (1987).
Li, G. et al. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 4, 864–868 (2005).
McCulloch, I. et al. Liquid-crystalline semiconducting polymers with high charge-carrier mobility. Nat. Mater. 5, 328–333 (2006).
Mohammadi, E. et al. Dynamic-template-directed multiscale assembly for large-area coating of highly-aligned conjugated polymer thin films. Nat. Commun. 8, 16070 (2017).
Kim, J. H. et al. Optimization and analysis of conjugated polymer side chains for high-performance organic photovoltaic cells. Adv. Func. Mater. 26, 1517–1525 (2016).
Abdelsamie, M. et al. Toward additive-free small-molecule organic solar cells: roles of the donor crystallization pathway and dynamics. Adv. Mater. 27, 7285–7292 (2015).
Gao, H. C., Xue, L. G., Xin, S. & Goodenough, J. B. A high-energy-density potassium battery with a polymer-gel electrolyte and a polyaniline cathode. Angew. Chem. Int. Ed. 57, 5449–5453 (2018).
Park, S. J. et al. Side-chain conducting and phase-separated polymeric binders for high-performance silicon anodes in lithium-ion batteries. J. Am. Chem. Soc. 137, 2565–2571 (2015).
Lee, M. J. et al. Elastomeric electrolytes for high-energy solid-state lithium batteries. Nature 601, 217–222 (2022).
Liu, G., Zheng, H., Song, X. & Battaglia, V. S. Particles and polymer binder interaction: a controlling factor in lithium-ion electrode performance. J. Electrochem. Soc. 159, A214–A221 (2012).
Chen, H. et al. Exploring chemical, mechanical, and electrical functionalities of binders for advanced energy–storage devices. Chem. Rev. 118, 8936–8982 (2018).
Zhao, H. et al. Toward practical application of functional conductive polymer binder for a high-energy lithium-ion battery design. Nano Lett. 14, 6704–6710 (2014).
Liu, K. et al. Thermotropic liquid crystals from biomacromolecules. Proc. Natl Acad. Sci. USA 111, 18596–18600 (2014).
Panova, O. et al. Diffraction imaging of nanocrystalline structures in organic semiconductor molecular thin films. Nat. Mater. 18, 860–865 (2019).
Fang, C. et al. Large-molecule decomposition products of electrolytes and additives revealed by on-electrode chromatography and MALDI. Joule 5, 415–428 (2021).
Chan, C. K. et al. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 3, 31–35 (2008).
Magasinski, A. et al. High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nat. Mater. 9, 353–358 (2010).
Wang, C. et al. Self-healing chemistry enables the stable operation of silicon microparticle anodes for high-energy lithium-ion batteries. Nat. Chem. 5, 1042–1048 (2013).
Choi, S., Kwon, T. W., Coskun, A. & Choi, J. W. Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries. Science 357, 279–283 (2017).
Chen, J. et al. Electrolyte design for LiF-rich solid-electrolyte interfaces to enable high-performance microsized alloy anodes for batteries. Nat. Energy 5, 386–397 (2020).
Tan, D. H. et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 373, 1494–1499 (2021).
Wu, S. X. et al. In-situ polymerized binder: a three-in-one design strategy for all-integrated SiOx anode with high mass loading in lithium ion batteries. ACS Energy Lett. 6, 290–297 (2021).
Jin, Y. et al. Understanding fluoroethylene carbonate and vinylene carbonate based electrolytes for Si anodes in lithium ion batteries with NMR spectroscopy. J. Am. Chem. Soc. 140, 9854–9867 (2018).
Zayat, B., Das, P., Thompson, B. C. & Narayan, S. R. In situ measurement of ionic and electronic conductivities of conductive polymers as a function of electrochemical doping in battery electrolytes. J. Phys. Chem. C. 125, 7533–7541 (2021).
Savitzky, B. H. et al. py4DSTEM: a software package for four-dimensional scanning transmission electron microscopy data analysis. Microsc. Microanal. 27, 712–743 (2021).
Acknowledgements
This work is primarily funded by the assistant secretary of energy efficiency in the Vehicle Technologies Office of the US Department of Energy under the I4-Lab programme. T.Z., C.F., Y.L., Y.F. and G.L. were supported by the Vehicle Technologies Office. H.S. and B.H.S. were supported by the Toyota Research Institute. Y.H., D.L., X.Z., C.O., C.Z., W.Y., A.M.M. and the Molecular Foundry and the Advanced Light Source were supported by the director of the Office of Science, Office of Basic Energy Sciences of the US Department of Energy under contract number DE-AC02-05CH11231.
Author information
Authors and Affiliations
Contributions
T.Z. and G.L. conceived and designed the experiments. C.O., C.Z., W.Y., A.M.M. and G.L. provided critical guidance on the experiments. H.S. provided important guidance on the TEM analysis and sample preparation. H.S. conducted the TEM experiments and 4D-STEM data collection. H.S. and B.H.S. conducted analysis of the 4D-STEM data. T.Z., Y.H., C.Z. and W.Y. performed the synchrotron-based measurements. D.L. performed mechanical testing. C.F. performed MALDI-TOF analysis. X.Z. performed atomic force microscopy and Raman spectroscopy for polymers. T.Z., Y.L and Y.F. performed battery fabrication and electrochemical testing. T.Z. performed all other experiments. T.Z. and G.L. wrote the first draft of the manuscript, and all the authors revised and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Ali Coskun, Rafael Verduzco and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Additional experimental details and Supplementary Notes 1–10, Figs. 1–39 and Tables 1–6.
Source data
Source Data Fig. 1
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, T., Sternlicht, H., Ha, Y. et al. Formation of hierarchically ordered structures in conductive polymers to enhance the performances of lithium-ion batteries. Nat Energy 8, 129–137 (2023). https://doi.org/10.1038/s41560-022-01176-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-022-01176-6
This article is cited by
-
High voltage electrolytes for lithium-ion batteries with micro-sized silicon anodes
Nature Communications (2024)
-
Influence of Polyaniline as Filler on the Microstructural Features and Properties of Polycarbonate: Bismuth Sulfide Nanocomposite
Journal of Inorganic and Organometallic Polymers and Materials (2024)
-
Heating up the binder
Nature Energy (2023)
-
Trimming the Degrees of Freedom via a K+ Flux Rectifier for Safe and Long-Life Potassium-Ion Batteries
Nano-Micro Letters (2023)