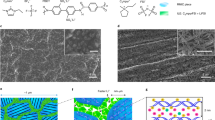
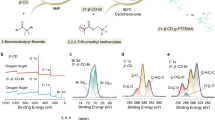
Abstract
Rational designs of solid polymer electrolytes with high ion conduction are critical in enabling the creation of advanced lithium batteries. However, known polymer electrolytes have much lower ionic conductivity than liquid/ceramics at room temperature, which limits their practical use in batteries. Here we show that precise positioning of designed repeating units in alternating polymer sequences lays the foundation for homogenized Li+ distribution, non-aggregated Li+-anion solvation and sequence-assisted site-to-site ion migration, facilitating the tuning of Li+ conductivity by up to three orders of magnitude. The assembled all-solid-state batteries facilitate reversible and dendrite-mitigated cycling against Li metal from ambient to elevated temperatures. This work demonstrates a powerful molecular engineering means to access highly ion-conductive solid-state materials for next-generation energy devices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source data are provided with this paper. Additional data supporting the findings of this study are included in the Supplementary Information.
References
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Albertus, P. et al. Challenges for and pathways toward Li-metal-based all-solid-state batteries. ACS Energy Lett. 6, 1399–1404 (2021).
Li, M., Lu, J., Chen, Z. & Amine, K. 30 Years of lithium-ion batteries. Adv. Mater. 30, 1800561 (2018).
Meng, Y. S., Srinivasan, V. & Xu, K. Designing better electrolytes. Science 378, 1065 (2022).
Hobold, G. M. et al. Moving beyond 99.9% Coulombic efficiency for lithium anodes in liquid electrolytes. Nat. Energy 6, 951–960 (2021).
Zhao, Q., Stalin, S., Zhao, C. Z. & Archer, L. A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 5, 229–252 (2020).
Banerjee, A., Wang, X., Fang, C., Wu, E. A. & Meng, Y. S. Interfaces and interphases in all-solid-state batteries with inorganic solid electrolytes. Chem. Rev. 120, 6878–6933 (2020).
Chen, R. S., Li, Q. H., Yu, X. Q., Chen, L. Q. & Li, H. Approaching practically accessible solid-state batteries: stability issues related to solid electrolytes and interfaces. Chem. Rev. 120, 6820–6877 (2020).
Lopez, J., Mackanic, D. G., Cui, Y. & Bao, Z. N. Designing polymers for advanced battery chemistries. Nat. Rev. Mater. 4, 312–330 (2019).
Li, Z. Y., Zhang, H. R., Sun, X. L. & Yang, Y. Mitigating interfacial instability in polymer electrolyte-based solid-state lithium metal batteries with 4 V cathodes. ACS Energy Lett. 5, 3244–3253 (2020).
Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4418 (2004).
Bachman, J. C. et al. Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem. Rev. 116, 140–162 (2016).
Bouchet, R. et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 12, 452–457 (2013).
Wang, X. et al. Ultra-stable all-solid-state sodium metal batteries enabled by perfluoropolyether-based electrolytes. Nat. Mater. 21, 1057–1065 (2022).
Ratner, M. A. & Shriver, D. F. Ion transport in solvent-free polymers. Chem. Rev. 88, 109–124 (1988).
Bocharova, V. & Sokolov, A. P. Perspectives for polymer electrolytes: a view from fundamentals of ionic conductivity. Macromolecules 53, 4141–4157 (2020).
Cheng, X. L., Pan, J., Zhao, Y., Liao, M. & Peng, H. S. Gel polymer electrolytes for electrochemical energy storage. Adv. Energy Mater. 8, 1702184 (2018).
Mindemark, J., Lacey, M. J., Bowden, T. & Brandell, D. Beyond PEO-alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 81, 114–143 (2018).
Wang, J. R., Li, S. Q., Zhao, Q., Song, C. & Xue, Z. G. Structure code for advanced polymer electrolyte in lithium‐ion batteries. Adv. Funct. Mater. 31, 2008208 (2020).
Qiao, B. et al. Supramolecular regulation of anions enhances conductivity and transference number of lithium in liquid electrolytes. J. Am. Chem. Soc. 140, 10932–10936 (2018).
Ma, Q. et al. Single lithium-ion conducting polymer electrolytes based on a super-delocalized polyanion. Angew. Chem. Inter. Ed. Engl. 55, 2521–2525 (2016).
Li, J. H. et al. Polymers in lithium-ion and lithium metal batteries. Adv. Energy Mater. 11, 2003239 (2021).
Zhang, H. et al. Single lithium-ion conducting solid polymer electrolytes: advances and perspectives. Chem. Soc. Rev. 46, 797–815 (2017).
Lu, Y. Y., Korf, K., Kambe, Y., Tu, Z. Y. & Archer, L. A. Ionic-liquid-nanoparticle hybrid electrolytes: applications in lithium metal batteries. Angew. Chem. Inter. Ed. Engl. 53, 488–492 (2014).
Doyle, M., Fuller, T. F. & Newman, J. The importance of the lithium ion transference number in lithium polymer cells. Electrochim. Acta 39, 2073–2081 (1994).
Li, S. P. et al. Single-ion homopolymer electrolytes with high transference number prepared by click chemistry and photoinduced metal-free atom-transfer radical polymerization. ACS Energy Lett. 3, 20–27 (2018).
Jangu, C. et al. Sulfonimide-containing triblock copolymers for improved conductivity and mechanical performance. Macromolecules 48, 4520–4528 (2015).
Lutz, J. F., Ouchi, M., Liu, D. R. & Sawamoto, M. Sequence-controlled polymers. Science 341, 1238149 (2013).
Barnes, J. C. et al. Iterative exponential growth of stereo- and sequence-controlled polymers. Nat. Chem. 7, 810–815 (2015).
Trigg, E. B. et al. Self-assembled highly ordered acid layers in precisely sulfonated polyethylene produce efficient proton transport. Nat. Mater. 17, 725–731 (2018).
Mao, G., Saboungi, M.-L., Price, D. L., Armand, M. B. & Howells, W. S. Structure of liquid PEO-LiTFSI electrolyte. Phys. Rev. Lett. 84, 5536–5539 (2000).
Makhlooghiazad, F. et al. Zwitterionic materials with disorder and plasticity and their application as non-volatile solid or liquid electrolytes. Nat. Mater. 21, 228–236 (2022).
Cowie, J. M. G. Alternating Copolymers (Plenum Press, 1985).
Corrigan, N. et al. Reversible-deactivation radical polymerization (controlled/living radical polymerization): from discovery to materials design and applications. Prog. Polym. Sci. 111, 101311 (2020).
Zhao, Y. et al. Controlled radical copolymerization of fluoroalkenes by using light-driven redox-relay catalysis. Nat. Synth. 2, 653–662 (2023).
Zhang, W. X. et al. Molecularly tunable polyanions for single-ion conductors and poly(solvate ionic liquids). Chem. Mater. 33, 524–534 (2021).
Atik, J. et al. Cation-assisted lithium-ion transport for high-performance PEO-based ternary solid polymer electrolytes. Angew. Chem. Inter. Ed. Engl. 60, 11919–11927 (2021).
Ma, M. Y. et al. Designing weakly solvating solid main-chain fluoropolymer electrolytes: synergistically enhancing stability toward Li anodes and high-voltage cathodes. ACS Energy Lett. 6, 4255–4264 (2021).
Nurnberg, P. et al. Superionicity in ionic-liquid-based electrolytes induced by positive ion-ion correlations. J. Am. Chem. Soc. 144, 4657–4666 (2022).
Huang, Y. F. et al. A relaxor ferroelectric polymer with an ultrahigh dielectric constant largely promotes the dissociation of lithium salts to achieve high ionic conductivity. Energ. Environ. Sci. 14, 6021–6029 (2021).
Chen, F., Wang, X., Armand, M. & Forsyth, M. Cationic polymer-in-salt electrolytes for fast metal ion conduction and solid-state battery applications. Nat. Mater. 21, 1175–1182 (2022).
Ue, M. & Mori, S. Mobility and ionic association of lithium salts in a propylene carbonate‐ethyl methyl carbonate mixed solvent. J. Electrochem. Soc. 142, 2577–2581 (1995).
Khurana, R., Schaefer, J. L., Archer, L. A. & Coates, G. W. Suppression of lithium dendrite growth using cross-linked polyethylene/poly(ethylene oxide) electrolytes: a new approach for practical lithium-metal polymer batteries. J. Am. Chem. Soc. 136, 7395–7402 (2014).
Bruce, P. G. & Vincent, C. A. Steady state current flow in solid binary electrolyte cells. J. Electroanal. Chem. 225, 1–17 (1987).
Wang, Y. et al. Solid-state rigid-rod polymer composite electrolytes with nanocrystalline lithium ion pathways. Nat. Mater. 20, 1255–1263 (2021).
Yu, Z. et al. A dynamic, electrolyte-blocking, and single-Ion-conductive network for stable lithium-metal anodes. Joule 3, 2761–2776 (2019).
Zhang, X. K. et al. Vertically aligned and continuous nanoscale ceramic-polymer interfaces in composite solid polymer electrolytes for enhanced ionic conductivity. Nano Lett. 18, 3829–3838 (2018).
Qing, X. et al. A functionalized metal organic framework-laden nanoporous polymer electrolyte for exceptionally stable lithium electrodeposition. Chem. Commun. 56, 15533–15536 (2020).
Lu, J. et al. The role of nanotechnology in the development of battery materials for electric vehicles. Nat. Nanotechnol. 11, 1031–1038 (2016).
Zhao, Y. et al. Organocatalyzed photoredox polymerization from aromatic sulfonyl halides: facilitating graft from aromatic C-H bonds. Macromolecules 51, 938–946 (2018).
Hess, B., Kutzner, C., van der Spoel, D. & Lindahl, E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008).
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (no. 21971044 to M.C. and 52003231 to X.L.), the Shanghai Pilot Program for Basic Research-Fudan University 21TQ1400100 (no. 21TQ007 to M.C.) and the State Key Laboratory of Molecular Engineering of Polymers and Duke Kunshan University. We thank J. A. Johnson for helpful discussions.
Author information
Authors and Affiliations
Contributions
S.H., X.L. and M.C. conceived the idea and designed the experiments. S.H. conducted major experiments and prepared the draft article. S.H. and X.L. contributed molecular dynamic simulations. P.W. and Y.Z. contributed DFT calculations. S.H., H.W., Y.G. and L.Z. conducted materials characterizations. S.H., Y.S.-H., X.L. and M.C. interpreted the data and completed the final manuscript writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Renaud Bouchet and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary discussion, Figs. 1–82 and Tables 1–17.
Source data
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, S., Wen, P., Wang, H. et al. Sequencing polymers to enable solid-state lithium batteries. Nat. Mater. 22, 1515–1522 (2023). https://doi.org/10.1038/s41563-023-01693-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-023-01693-z
This article is cited by
-
Electrochemical benefits of conductive polymers as a cathode material in LFP battery technology
Journal of Solid State Electrochemistry (2024)