Abstract
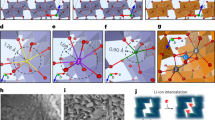
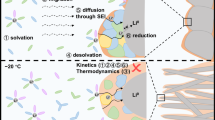
High-rate cathode materials for Li-ion batteries require fast Li transport kinetics, which typically rely on topotactic Li intercalation/de-intercalation because it minimally disrupts Li transport pathways. In contrast to this conventional view, here we demonstrate that the rate capability in a Li-rich cation-disordered rocksalt cathode can be significantly improved when the topotactic reaction is replaced by a non-topotactic reaction. The fast non-topotactic lithiation reaction is enabled by facile and reversible transition metal octahedral-to-tetrahedral migration, which improves rather than impedes Li transport. Using this concept, we show that high-rate performance can be achieved in Mn- and Ni-based cation-disordered rocksalt materials when some of the transition metal content can reversibly switch between octahedral and tetrahedral sites. This study provides a new perspective on the design of high-performance cathode materials by demonstrating how the interplay between Li and transition metal migration in materials can be conducive to fast non-topotactic Li intercalation/de-intercalations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated and analysed during this study are included in the published article and its Supplementary Information. Source data are provided with this paper.
References
Kang, K., Meng, Y. S., Bréger, J., Grey, C. P. & Ceder, G. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 311, 977–980 (2006).
Schougaard, S. B., Bréger, J., Jiang, M., Grey, C. P. & Goodenough, J. B. LiNi0.5+δMn0.5–δO2—A high-rate, high-capacity cathode for lithium rechargeable batteries. Adv. Mater. 18, 905–909 (2006).
Julien, C. & Nazri, G. A. in Handbook of Advanced Electronic and Photonic Materials and Devices (ed. Singh Nalwa, H.) Ch. 3 (Academic Press, 2001).
Jacobson, A. J. & Nazar, L. F. Intercalation Chemistry. Encyclopedia of Inorganic and Bioinorganic Chemistry https://doi.org/10.1002/9781119951438.eibc0093 (2011).
Wu, F. & Yushin, G. Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ. Sci. 10, 435–459 (2017).
Wiaderek, K. M. et al. Comprehensive insights into the structural and chemical changes in mixed-anion FeOF electrodes by using operando PDF and NMR spectroscopy. J. Am. Chem. Soc. 135, 4070–4078 (2013).
Yu, H.-C. et al. Designing the next generation high capacity battery electrodes. Energy Environ. Sci. 7, 1760–1768 (2014).
Huang, Q. et al. Fading mechanisms and voltage hysteresis in FeF2–NiF2 solid solution cathodes for lithium and lithium-ion batteries. Small 15, 1804670 (2019).
Hua, X. et al. Comprehensive study of the CuF2 conversion reaction mechanism in a lithium ion battery. J. Phys. Chem. C 118, 15169–15184 (2014).
Bréger, J. et al. Effect of high voltage on the structure and electrochemistry of LiNi0.5Mn0.5O2: a joint experimental and theoretical study. Chem. Mater. 18, 4768–4781 (2006).
Li, H. H. et al. Changes in the cation ordering of layered O3 LixNi0.5Mn0.5O2 during electrochemical cycling to high voltages: an electron diffraction study. Chem. Mater. 19, 2551–2565 (2007).
Jones, C. D. W., Rossen, E. & Dahn, J. R. Structure and electrochemistry of LixCryCo1−yO2. Solid State Ion. 68, 65–69 (1994).
Lyu, Y. et al. Atomic insight into electrochemical inactivity of lithium chromate (LiCrO2): irreversible migration of chromium into lithium layers in surface regions. J. Power Sources 273, 1218–1225 (2015).
Lee, J. et al. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science 343, 519–522 (2014).
Clément, R. J., Lun, Z. & Ceder, G. Cation-disordered rocksalt transition metal oxides and oxyfluorides for high energy lithium-ion cathodes. Energy Environ. Sci. 13, 345–373 (2020).
Balasubramanian, M., McBreen, J., Davidson, I. J., Whitfield, P. S. & Kargina, I. In situ X-ray absorption study of a layered manganese-chromium oxide-based cathode material. J. Electrochem. Soc. 149, A176–A184 (2002).
Yang, W. & Devereaux, T. P. Anionic and cationic redox and interfaces in batteries: advances from soft X-ray absorption spectroscopy to resonant inelastic scattering. J. Power Sources 389, 188–197 (2018).
Dai, K. et al. High reversibility of lattice oxygen redox quantified by direct bulk probes of both anionic and cationic redox reactions. Joule 3, 518–541 (2019).
Li, N. et al. Correlating the phase evolution and anionic redox in Co-free Ni-rich layered oxide cathodes. Nano Energy 78, 105365 (2020).
Papp, J. K. et al. A comparison of high voltage outgassing of LiCoO2, LiNiO2, and Li2MnO3 layered Li-ion cathode materials. Electrochim. Acta 368, 137505 (2021).
Renfrew, S. E. & McCloskey, B. D. Residual lithium carbonate predominantly accounts for first cycle CO2 and CO outgassing of Li-stoichiometric and Li-rich layered transition-metal oxides. J. Am. Chem. Soc. 139, 17853–17860 (2017).
Renfrew, S. E. & McCloskey, B. D. Quantification of surface oxygen depletion and solid carbonate evolution on the first cycle of LiNi0.6Mn0.2Co0.2O2 electrodes. ACS Appl. Energy Mater. 2, 3762–3772 (2019).
Van der Ven, A. & Ceder, G. Lithium diffusion mechanisms in layered intercalation compounds. J. Power Sources 97–98, 529–531 (2001).
Van der Ven, A. Lithium diffusion in layered LixCoO. Electrochem. Solid-State Lett. 3, 301 (1999).
Urban, A., Lee, J. & Ceder, G. The configurational space of rocksalt-type oxides for high-capacity lithium battery electrodes. Adv. Energy Mater. 4, 1400478 (2014).
Reed, J. & Ceder, G. Role of electronic structure in the susceptibility of metastable transition-metal oxide structures to transformation. Chem. Rev. 104, 4513–4534 (2004).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976).
Ji, H. et al. Hidden structural and chemical order controls lithium transport in cation-disordered oxides for rechargeable batteries. Nat. Commun. 10, 592 (2019).
Nakajima, M. & Yabuuchi, N. Lithium-excess cation-disordered rocksalt-type oxide with nanoscale phase segregation: Li1.25Nb0.25V0.5O2. Chem. Mater. 29, 6927–6935 (2017).
Zhu, Z. et al. Gradient Li-rich oxide cathode particles immunized against oxygen release by a molten salt treatment. Nat. Energy 4, 1049–1058 (2019).
Wang, Y., Yang, Z., Qian, Y., Gu, L. & Zhou, H. New insights into improving rate performance of lithium-rich cathode material. Adv. Mater. 27, 3915–3920 (2015).
Shi, J.-L. et al. High-capacity cathode material with high voltage for Li-ion batteries. Adv. Mater. 30, 1705575 (2018).
Li, X. et al. A new type of Li-rich rock-salt oxide Li2Ni1/3Ru2/3O3 with reversible anionic redox chemistry. Adv. Mater. 31, 1807825 (2019).
Hong, J. et al. Metal–oxygen decoordination stabilizes anion redox in Li-rich oxides. Nat. Mater. 18, 256–265 (2019).
Kang, K. & Ceder, G. Factors that affect Li mobility in layered lithium transition metal oxides. Phys. Rev. B 74, 94105 (2006).
Sathiya, M. et al. Origin of voltage decay in high-capacity layered oxide electrodes. Nat. Mater. 14, 230–238 (2015).
Bréger, J., Kang, K., Cabana, J., Ceder, G. & Grey, C. P. NMR, PDF and RMC study of the positive electrode material Li(Ni0.5Mn0.5)O2 synthesized by ion-exchange methods. J. Mater. Chem. 17, 3167–3174 (2007).
Sun, X., Duffort, V., Mehdi, B. L., Browning, N. D. & Nazar, L. F. Investigation of the mechanism of Mg insertion in birnessite in nonaqueous and aqueous rechargeable Mg-ion batteries. Chem. Mater. 28, 534–542 (2016).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Ravel, B. & Newville, M. ATHENA and ARTEMIS: interactive graphical data analysis using IFEFFIT. Phys. Scr. 15, 1007–1010 (2005).
Qiao, R. et al. High-efficiency in situ resonant inelastic X-ray scattering (iRIXS) endstation at the advanced light source. Rev. Sci. Instrum. 88, 33106 (2017).
Wu, J. et al. Elemental-sensitive detection of the chemistry in batteries through soft X-ray absorption spectroscopy and resonant inelastic X-ray scattering. JoVE 134, e57415 (2018).
McCloskey, B. D., Bethune, D. S., Shelby, R. M., Girishkumar, G. & Luntz, A. C. Solvents’ critical role in nonaqueous lithium-oxygen battery electrochemistry. J. Phys. Chem. Lett. 2, 1161–1166 (2011).
Mccloskey, B. D. et al. Combining accurate O2 and Li2O2 assays to separate discharge and charge stability limitations in nonaqueous Li–O2 Batteries. J. Phys. Chem. Lett. 4, 2989–2993 (2013).
McCloskey, B. D. et al. Twin problems of interfacial carbonate formation in nonaqueous Li–O2 batteries. J. Phys. Chem. Lett. 3, 997–1001 (2012).
Van der Ven, A., Aydinol, M. K., Ceder, G., Kresse, G. & Hafner, J. First-principles investigation of phase stability in LixCoO2. Phys. Rev. B 58, 2975–2987 (1998).
van de Walle, A. Multicomponent multisublattice alloys, nonconfigurational entropy and other additions to the Alloy Theoretic Automated Toolkit. Calphad 33, 266–278 (2009).
Nelson, L. J., Hart, G. L. W., Zhou, F. & Ozoliņš, V. Compressive sensing as a paradigm for building physics models. Phys. Rev. B 87, 35125 (2013).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Wang, L., Maxisch, T. & Ceder, G. Oxidation energies of transition metal oxides within the GGA-U framework. Phys. Rev. B 73, 195107 (2006).
Jain, A. et al. Formation enthalpies by mixing GGA and GGA + U calculations. Phys. Rev. B 84, 45115 (2011).
Reed, J., Ceder, G. & Van Der Ven, A. Layered-to-spinel phase transition in LixMnO2. Electrochem. Solid-State Lett. 4, A78 (2001).
Furness, J. W., Kaplan, A. D., Ning, J., Perdew, J. P. & Sun, J. Accurate and numerically efficient r2SCAN meta-generalized gradient approximation. J. Phys. Chem. Lett. 11, 8208–8215 (2020).
Sun, J., Ruzsinszky, A. & Perdew, J. P. Strongly constrained and appropriately normed semilocal density functional. Phys. Rev. Lett. 115, 36402 (2015).
Kitchaev, D. A. et al. Energetics of MnO2 polymorphs in density functional theory. Phys. Rev. B 93, 45132 (2016).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Urban, A., Seo, D.-H. & Ceder, G. Computational understanding of Li-ion batteries. NPJ Comput. Mater. 2, 16002 (2016).
Asari, Y., Suwa, Y. & Hamada, T. Formation and diffusion of vacancy-polaron complex in olivine-type LiMnPO4 and LiFePO4. Phys. Rev. B 84, 134113 (2011).
Acknowledgements
This work was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Vehicle Technologies Office, under the Applied Battery Materials Program of the US Department of Energy under contract no. DE-AC02-05CH11231. The XAS measurements were performed at the Advanced Photon Source at Argonne National Laboratory, which is supported by the US Department of Energy under contract no. DE-AC02-06CH11357. Work at the Advanced Light Source was supported by the US DOE Office of Science User Facility under contract no. DE-AC02-05CH11231. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under contract no. DE-AC02-05CH11231. The computational analysis was performed using computational resources sponsored by the US Department of Energy’s Office of Energy Efficiency and Renewable Energy and located at the National Renewable Energy Laboratory, as well as computational resources provided by the Extreme Science and Engineering Discovery Environment (XSEDE), supported by National Science Foundation grant no. ACI1053575, and the National Energy Research Scientific Computing Center (NERSC), a DOE Office of Science User Facility supported by the Office of Science and the US Department of Energy under contract no. DE-AC02-05CH11231. We thank H. Kim and Z. Lun for assistance with the XAS measurements.
Author information
Authors and Affiliations
Contributions
J.H. and G.C. planned the project. G.C. supervised all aspects of the research. J.H. synthesized, characterized and electrochemically tested the proposed materials. With help from M.B., J.H. also analysed the ex situ XAS data. P.Z. performed the theoretical calculations and analysed the results. D.-H.K. performed the TEM characterization. Y.H. collected and analysed the RIXS data with W.Y. With input from B.D.M., M.J.C. collected and analysed the DEMS data. Y.T. performed the SEM characterization. The manuscript was written by J.H. and G.C. and was revised by the other co-authors. All the authors contributed to discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Energy thanks Naoaki Yabuuchi, Yujie Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19, Tables 1 and 2, Notes 1–6 and references.
Source data
Source Data Fig. 1
XRD data.
Source Data Fig. 2
Electrochemical performance.
Source Data Fig. 3
XAS at Mn and Cr K-edge.
Source Data Fig. 5
XRD, electrochemistry, XAS and RIXS of LNTO and LNTC02O.
Rights and permissions
About this article
Cite this article
Huang, J., Zhong, P., Ha, Y. et al. Non-topotactic reactions enable high rate capability in Li-rich cathode materials. Nat Energy 6, 706–714 (2021). https://doi.org/10.1038/s41560-021-00817-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-021-00817-6
This article is cited by
-
Engineering the micro/nano structure of Ca3Co4O9 anode material for lithium-ion batteries
Journal of Materials Science: Materials in Electronics (2024)
-
Inhibiting collective cation migration in Li-rich cathode materials as a strategy to mitigate voltage hysteresis
Nature Materials (2023)
-
Steady-state interface construction of high-voltage nickel-rich lithium-ion battery cathodes by low-content LixCoO2 surface modification engineering
Ionics (2023)
-
Transition metal migration and O2 formation underpin voltage hysteresis in oxygen-redox disordered rocksalt cathodes
Nature Communications (2022)
-
Approaches for handling high-dimensional cluster expansions of ionic systems
npj Computational Materials (2022)