Abstract
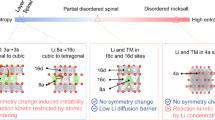
The lithium-excess manganese oxides are a candidate cathode material for the next generation of Li-ion batteries because of their ability to reversibly intercalate more Li than traditional cathode materials. Although reversible oxidation of lattice oxygen has been proposed as the origin of this anomalous excess capacity, questions about the underlying electrochemical reaction mechanisms remain unresolved. Here, we critically analyse the O2−/O− oxygen redox hypothesis and explore alternative explanations for the origin of the anomalous capacity, including the formation of peroxide ions or trapped oxygen molecules and the oxidation of Mn. First-principles calculations motivated by the Li–Mn–O phase diagram show that the electrochemical behaviour of the Li-excess manganese oxides is thermodynamically consistent with the oxidation of Mn from the +4 oxidation state to the +7 oxidation state and the concomitant migration of Mn from octahedral sites to tetrahedral sites. It is shown that the Mn oxidation hypothesis can explain the poorly understood electrochemical behaviour of Li-excess materials, including the activation step, the voltage hysteresis and voltage fade.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The analysis presented here can be reproduced with the data provided in the paper, supporting information and cited references. Additional calculation data generated during this study are available upon reasonable request.
References
Lu, Z., Beaulieu, L. Y., Donaberger, R. A., Thomas, C. L. & Dahn, J. R. Synthesis, structure, and electrochemical behavior of Li[NixLi1/3−2x/3Mn2/3−x/3]O2. J. Electrochem. Soc. 149, A778–A791 (2002).
Johnson, C. S. et al. The significance of the Li2MnO3 component in ‘composite’ xLi2MnO3 · (1−x)LiMn0.5Ni 0.5O2 electrodes. Electrochem. Commun. 6, 1085–1091 (2004).
Hong, J., Gwon, H., Jung, S.-K., Ku, K. & Kang, K. Review: Lithium-excess layered cathodes for lithium rechargeable batteries. J. Electrochem. Soc. 162, A2447–A2467 (2015).
Hy, S. et al. Performance and design considerations for the lithium excess layered oxide positive electrode materials for lithium ion batteries. Energy Environ. Sci. 9, 1931–1954 (2016).
Radin, M. D. et al. Narrowing the gap between theoretical and practical capacities in Li-ion layered oxide cathode materials. Adv. Energy Mater. 7, 1602888 (2017).
Wei, Y. J. et al. Electrochemical kinetics and cycling performance of nano Li[Li0.23Co0.3Mn0.47]O2 cathode material for lithium ion batteries. Electrochem. Commun. 11, 2008–2011 (2009).
Johnson, C. S., Li, N., Lefief, C., Vaughey, J. T. & Thackeray, M. M. Synthesis, characterization and electrochemistry of lithium battery electrodes: xLi2MnO3 · (1−x)LiMn0.333Ni0.333Co0.333O2 (0 ≤ x ≤ 0.7). Chem. Mater. 20, 6095–6106 (2008).
Bettge, M. et al. Voltage fade of layered oxides: its measurement and impact on energy density. J. Electrochem. Soc. 160, A2046–A2055 (2013).
Kalyani, P., Chitra, S., Mohan, T. & Gopukumar, S. Lithium metal rechargeable cells using Li2MnO3 as the positive electrode. J. Power Sources 80, 103–106 (1999).
Robertson, A. D. & Bruce, P. G. Mechanism of electrochemical activity in Li2MnO3. Chem. Mater. 15, 1984–1992 (2003).
Yu, D. Y. W., Yanagida, K., Kato, Y. & Nakamura, H. Electrochemical activities in Li2MnO3. J. Electrochem. Soc. 156, A417–A424 (2009).
Park, Y. J. et al. Synthesis and electrochemical characteristics of Li[CoxLi(1/3−x/3)Mn(2/3−2x/3)]O2 compounds. J. Electrochem. Soc. 151, A720–A727 (2004).
Yabuuchi, N. et al. High-capacity electrode materials for rechargeable lithium batteries: Li3NbO4-based system with cation-disordered rocksalt structure. Proc. Natl Acad. Sci. USA 112, 7650–7655 (2015).
Yabuuchi, N. et al. Origin of stabilization and destabilization in solid-state redox reaction of oxide ions for lithium-ion batteries. Nat. Commun. 7, 13814 (2016).
Yabuuchi, N. et al. A new electrode material for rechargeable sodium batteries: P2-type Na2/3[Mg0.28Mn0.72]O2 with anomalously high reversible capacity. J. Mater. Chem. A 2, 16851–16855 (2014).
Maitra, U. et al. Oxygen redox chemistry without excess alkali-metal ions in Na2/3[Mg0.28Mn0.72]O2. Nat. Chem. 10, 288–295 (2018).
Mortemard de Boisse, B. et al. Highly reversible oxygen-redox chemistry at 4.1 V in Na4/7−x[ϒ1/7Mn6/7]O2 (ϒ: Mn vacancy). Adv. Energy Mater. 2, 1800409 (2018).
Bai, X. et al. Anionic redox activity in a newly Zn-doped sodium layered oxide P2-Na2/3Mn1−yZnyO2 (0 < y < 0.23). Adv. Energy Mater. 8, 1802379 (2018).
Sathiya, M. et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 12, 827–835 (2013).
Hong, J. et al. Metal–oxygen decoordination stabilizes anion redox in Li-rich oxides. Nat. Mater. 18, 256–265 (2019).
Pearce, P. E. et al. Evidence for anionic redox activity in a tridimensional-ordered Li-rich positive electrode β-Li2IrO3. Nat. Mater. 16, 580–586 (2017).
McCalla, E. et al. Visualization of O–O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science 350, 1516–1521 (2015).
Saubanère, M., McCalla, E., Tarascon, J.-M. & Doublet, M.-L. The intriguing question of anionic redox in high-energy density cathodes for Li-ion batteries. Energy Environ. Sci. 9, 984–991 (2016).
Assat, G. & Tarascon, J.-M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat. Energy 3, 373–386 (2018).
Seo, D. et al. The electronic origin of the oxygen redox activity in Li-excess cathode materials. Nat. Chem. 8, 692–697 (2016).
Luo, K. et al. Charge-compensation in 3 d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen. Nat. Chem. 8, 684–691 (2016).
Koga, H. et al. Different oxygen redox participation for bulk and surface: a possible global explanation for the cycling mechanism of Li1.20Mn0.54Co0.13Ni0.13O2. J. Power Sources 236, 250–258 (2013).
Koga, H. et al. Operando X-ray absorption study of the redox processes involved upon cycling of the Li-rich layered oxide Li1.20Mn0.54Co0.13Ni0.13O2 in Li ion batteries. J. Phys. Chem. C. 118, 5700–5709 (2014).
Koyama, Y., Tanaka, I., Nagao, M. & Kanno, R. First-principles study on lithium removal from Li2MnO3. J. Power Sources 189, 798–801 (2009).
Gent, W. E. et al. Coupling between oxygen redox and cation migration explains unusual electrochemistry in lithium-rich layered oxides. Nat. Commun. 8, 2091 (2017).
Xu, J. et al. Elucidating anionic oxygen activity in lithium-rich layered oxides. Nat. Commun. 9, 947 (2018).
Kubobuchi, K. et al. Mn L2,3-edge X-ray absorption spectroscopic studies on charge–discharge mechanism of Li2MnO3. Appl. Phys. Lett. 104, 053906 (2014).
Rana, J. et al. Structural changes in Li2MnO3 cathode material for Li-ion batteries. Adv. Energy Mater. 4, 1300998 (2014).
Oishi, M. et al. Direct observation of reversible charge compensation by oxygen ion in Li-rich manganese layered oxide positive electrode material, Li1.16Ni0.15Co0.19Mn0.50O2. J. Power Sources 276, 89–94 (2015).
Tran, N. et al. Mechanisms associated with the ‘plateau’ observed at high voltage for the overlithiated Li1.12Ni0.425Mn 0.425Co0.15)0.88O2 system. Chem. Mater. 20, 4815–4825 (2008).
Armstrong, A. R. et al. Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2. J. Am. Chem. Soc. 128, 8694–8698 (2006).
Lee, E. & Persson, K. A. Structural and chemical evolution of the layered Li-excess LixMnO3 as a function of Li content from first-principles calculations. Adv. Energy Mater. 4, 1400498 (2014).
Malik, R., Abdellahi, A. & Ceder, G. A Critical review of the Li insertion mechanisms in LiFePO4 electrodes. J. Electrochem. Soc. 160, A3179–A3197 (2013).
Rinaldo, S. G. et al. Physical theory of voltage fade in lithium- and manganese-rich transition metal oxides. J. Electrochem. Soc. 162, A897–A904 (2015).
Zhuo, Z. et al. Spectroscopic signature of oxidized oxygen states in peroxides. J. Phys. Chem. Lett. 9, 6378–6384 (2018).
Glans, P. et al. Resonant X-ray emission spectroscopy of molecular oxygen. Phys. Rev. Lett. 76, 2448–2451 (1996).
Lebens-Higgins, Z. W. et al. Distinction between intrinsic and X-ray induced oxidized oxygen states in Li-rich 3 d layered oxides and LiAlO2. J. Phys. Chem. C 123, 13201–13207 (2019).
Bercx, M., Slap, L., Partoens, B. & Lamoen, D. First-principles investigation of the stability of the oxygen framework of Li-rich battery cathodes. MRS Adv. 4, 813–820 (2017).
Chen, H. & Islam, M. S. Lithium extraction mechanism in Li-rich Li2MnO3 involving oxygen hole formation and dimerization. Chem. Mater. 28, 6656–6663 (2016).
Li, X. et al. Direct visualization of the reversible O2−/O− redox process in Li-rich cathode materials. Adv. Mater. 30, 1705197 (2018).
Van der Ven, A., Aydinol, M. K., Ceder, G., Kresse, G. & Hafner, J. First-principles investigation of phase stability in LixCoO2. Phys. Rev. B 58, 2975–2987 (1998).
Wolverton, C. & Zunger, A. First-principles prediction of vacancy order–disorder and intercalation battery voltages in LixCoO2. Phys. Rev. Lett. 81, 606–609 (1998).
Qiu, B., Zhang, M., Xia, Y., Liu, Z. & Meng, Y. S. Understanding and controlling anionic electrochemical activity in high-capacity oxides for next generation Li-ion batteries. Chem. Mater. 29, 908–915 (2017).
Okubo, M. & Yamada, A. Molecular orbital principles of oxygen-redox battery electrodes. ACS Appl. Mater. Interfaces 9, 36463–36472 (2017).
Li, B. & Xia, D. Anionic redox in rechargeable lithium batteries. Adv. Mater. 29, 1701054 (2017).
Schirmer, O. F. Smoky coloration of quartz caused by bound small hole polaron optical absorption. Solid State Commun. 18, 1349–1351 (1976).
Griscom, D. L. Self-trapped holes in pure-silica glass: a history of their discovery and characterization and an example of their critical significance to industry. J. Non Cryst. Solids 352, 2601–2617 (2006).
Grimaud, A., Hong, W. T., Shao-Horn, Y. & Tarascon, J.-M. Anionic redox processes for electrochemical devices. Nat. Mater. 15, 121–126 (2016).
Lee, P. A., Nagaosa, N. & Wen, X. G. Doping a Mott insulator: physics of high-temperature superconductivity. Rev. Mod. Phys. 78, 17–85 (2006).
Subedi, A., Peil, O. E. & Georges, A. Low-energy description of the metal–insulator transition in the rare-earth nickelates. Phys. Rev. B 91, 075128 (2015).
Hu, E. et al. Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat. Energy 3, 690–698 (2018).
Yang, W. & Devereaux, T. P. Anionic and cationic redox and interfaces in batteries: advances from soft X-ray absorption spectroscopy to resonant inelastic scattering. J. Power Sources 389, 188–197 (2018).
Dai, K. et al. High reversibility of lattice oxygen redox in Na-ion and Li-ion batteries quantified by direct bulk probes of both anionic and cationic redox reactions. Joule 3, 518–541 (2019).
Chan, M. K. Y. et al. Structure of lithium peroxide. J. Phys. Chem. Lett. 2, 2483–2486 (2011).
Wang, L., Maxisch, T. & Ceder, G. A first-principles approach to studying the thermal stability of oxide cathode materials. Chem. Mater. 19, 543–552 (2007).
Yabuuchi, N., Yoshii, K., Myung, S. T., Nakai, I. & Komaba, S. Detailed studies of a high-capacity electrode material for rechargeable batteries, Li2MnO3–LiCo1/3Ni1/3Mn1/3O2. J. Am. Chem. Soc. 133, 4404–4419 (2011).
Lu, Z. & Dahn, J. R. Understanding the anomalous capacity of Li/Li[NixLi(1/3−2x/3)Mn(2/3−x/3)]O2 cells using in situ X-ray diffraction and electrochemical studies. J. Electrochem. Soc. 149, A815–A822 (2002).
Pasero, D., McLaren, V., De Souza, S. & West, A. R. Oxygen nonstoichiometry in Li2MnO3: an alternative explanation for its anomalous electrochemical activity. Chem. Mater. 17, 345–348 (2005).
Kubota, K. et al. Direct synthesis of oxygen-deficient Li2MnO3−x for high capacity lithium battery electrodes. J. Power Sources 216, 249–255 (2012).
Okamoto, Y. Ambivalent effect of oxygen vacancies on Li2MnO3: a first-principles study. J. Electrochem. Soc. 159, A152–A157 (2012).
Armstrong, A. R., Robertson, A. D. & Bruce, P. G. Overcharging manganese oxides: extracting lithium beyond Mn4+. J. Power Sources 146, 275–280 (2005).
Ohzuku, T., Nagayama, M., Tsuji, K. & Ariyoshi, K. High-capacity lithium insertion materials of lithium nickel manganese oxides for advanced lithium-ion batteries: toward rechargeable capacity more than 300 mA h g−1. J. Mater. Chem. 21, 10179 (2011).
Jain, A. et al. Commentary. The materials project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Ong, S. P., Wang, L., Kang, B. & Ceder, G. Li–Fe–P–O2 phase diagram from first principles calculations. Chem. Mater. 77, 1798–1807 (2008).
Jain, A. et al. Formation enthalpies by mixing GGA and GGA + U calculations. Phys. Rev. B 84, 045115 (2011).
Strehle, B. et al. The role of oxygen release from Li- and Mn-rich layered oxides during the first cycles investigated by on-line electrochemical mass spectrometry. J. Electrochem. Soc. 164, A400–A406 (2017).
Thackeray, M. M., Chan, M. K. Y., Trahey, L., Kirklin, S. & Wolverton, C. Vision for designing high-energy, hybrid Li ion/Li−O2 cells. J. Phys. Chem. Lett. 4, 3607–3611 (2013).
Ruther, R. E., Callender, A. F., Zhou, H., Martha, S. K. & Nanda, J. Raman microscopy of lithium-manganese-rich transition metal oxide cathodes. J. Electrochem. Soc. 162, A98–A102 (2014).
Thackeray, M. M. Manganese oxides for lithium batteries. Prog. Solid State Chem. 25, 1–71 (1997).
Jansen, V. M. & Hoppe, R. Zur Kenntnis der NaCl-Strukturfamilie: Neue Untersuchungen an Li2MnO3. Z. Anorg. Allg. Chem. 397, 279–289 (1973).
Fischer, D., Hoppe, R., Schäfer, W. & Knight, K. S. Koordinationszahl 4 oder 6 für Lithium? Die Kristallstruktur von wasserfreiem Lithiumpermanganat, Li[MnO4]. Z. Anorg. Allg. Chem. 619, 1419–1425 (1993).
Kitchaev, D. A. et al. Energetics of MnO2 polymorphs in density functional theory. Phys. Rev. B 93, 045132 (2016).
Chevrier, V. L., Ong, S. P., Armiento, R., Chan, M. K. Y. & Ceder, G. Hybrid density functional calculations of redox potentials and formation energies of transition metal compounds. Phys. Rev. B 82, 075122 (2010).
Aykol, M., Dwaraknath, S. S., Sun, W. & Persson, K. A. Thermodynamic limit for synthesis of metastable inorganic materials. Sci. Adv. 4, eaaq0148 (2018).
Chase, M. W. Jr. NIST-JANAF Thermochemical Tables (American Chemical Society, 1998).
Pauling, L. The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry (Cornell Univ. Press, 1960).
Zheng, J. et al. Electrochemical kinetics and performance of layered composite cathode material Li[Li0.2Ni0.2Mn0.6]O2. J. Electrochem. Soc. 160, 2212–2219 (2013).
Yu, H.-C. et al. Designing the next generation high capacity battery electrodes. Energy Environ. Sci. 7, 1760–1768 (2014).
Li, V. Von & Hoppe, V. G. M. R. Zum thermischen Verhalten von Li3MnO4 I. 1. Über α‐ und β‐Li3MnO4. 266, 249–256 (1976).
Ammundsen, B. et al. Local structure and first cycle redox mechanism of layered Li1.2Cr0.4Mn0.4O2 cathode material. J. Electrochem. Soc. 149, A431–A436 (2002).
Balasubramanian, M., McBreen, J., Davidson, I. J., Whitfield, P. S. & Kargina, I. In situ X-ray absorption study of a layered manganese-chromium oxide-based cathode material. J. Electrochem. Soc. 149, A176–A184 (2002).
Lu, Z. & Dahn, J. R. In situ and ex situ XRD investigation of Li[CrxLi1/3−x/3Mn2/3−2x/3]O2 (x = 1/3) cathode material. J. Electrochem. Soc. 150, A1044–A1051 (2003).
Na, Y., Ho, S., Chan, Y. & Bin, S. Characteristics of Li2TiO3–LiCrO2 composite cathode powders prepared by ultrasonic spray pyrolysis. J. Power Sources 244, 336–343 (2013).
Chalmin, E., Farges, F. & Brown, G. E. A pre-edge analysis of Mn K-edge XANES spectra to help determine the speciation of manganese in minerals and glasses. Contrib. Mineral. Petrol. 157, 111–126 (2009).
Van Schooneveld, M. M. & DeBeer, S. A close look at dose: toward L-edge XAS spectral uniformity, dose quantification and prediction of metal ion photoreduction. J. Electron Spectrosc. 198, 31–56 (2015).
Garvie, L. A. J. & Craven, A. J. High-resolution parallel electron energy-loss spectroscopy of Mn L2, 3-edges in inorganic manganese compounds. Phys. Chem. Miner. 21, 191–206 (1994).
Minasian, S. G. et al. Covalency in metal–oxygen multiple bonds evaluated using oxygen K-edge spectroscopy and electronic structure theory. J. Am. Chem. Soc. 135, 1864–1871 (2013).
Kleiner, K. et al. Origin of high capacity and poor cycling stability of Li-rich layered oxides — a long-duration in situ synchrotron powder diffraction study. Chem. Mater. 30, 3656–3667 (2018).
Kiefer, W. & Bernstein, H. J. Rotating Raman sample technique for colored crystal powders; resonance Raman effect in solid KMnO4. Appl. Spectrosc. 25, 609–613 (1971).
Du, K. et al. Exploring reversible oxidation of oxygen in a manganese oxide. Energy Environ. Sci. 9, 2575–2577 (2016).
Ben Yahia, M., Vergnet, J., Saubanère, M. & Doublet, M. L. Unified picture of anionic redox in Li/Na-ion batteries. Nat. Mater. 18, 496–502 (2019).
Qiao, Y. et al. Reversible anionic redox activity in Na3RuO4 cathodes: a prototype Na-rich layered oxide. Energy Environ. Sci. 11, 299–305 (2018).
Griffith, W. P. Infrared spectra of tetrahedral oxyanions of the transition metals. J. Chem. Soc. A 1467–1468 (1966).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Heyd, J., Scuseria, G. E. & Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 118, 8207–8215 (2003).
Krukau, A. V., Vydrov, O. A., Izmaylov, A. F. & Scuseria, G. E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 125, 224106 (2006).
Dudarev, S., Botton, G., Savrasov, S., Humphreys, C. & Sutton, A. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Blöchl, P., Jepsen, O. & Andersen, O. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 49, 16223–16233 (1994).
Chernova, N. A. et al. What can we learn about battery materials from their magnetic properties? J. Mater. Chem. 21, 9865–9875 (2011).
Acknowledgements
We thank L. Piper and Z. Lebens-Higgins for the insightful discussion. This work was supported as part of the NorthEast Center for Chemical Energy Storage (NECCES), an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences under award no. DE- SC0012583. The contributions of R.S. were supported as part of the Center for Synthetic Control Across Length-scales for Advancing Rechargeables (SCALAR), an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences under award no. DE-SC0019381. This research used resources of the National Energy Research Scientific Computing Center (NERSC), a US Department of Energy Office of Science User Facility operated under contract no. DE-AC02-05CH11231. Use of the Center for Scientific Computing at UC Santa Barbara supported by the National Science Foundation (NSF) Materials Research Science and Engineering Centers program through NSF DMR 1720256 and NSF CNS 1725797 is also acknowledged.
Author information
Authors and Affiliations
Contributions
All of the authors participated in the analysis of data and preparation of the manuscript. First-principles calculations were performed by M.D.R. and J.V.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–2, Supplementary Table 1, Supplementary Discussion and Supplementary refs.
Supplementary Data 1
Hypothesized crystal structure for Li1/2MnO3, in VASP POSCAR format.
Rights and permissions
About this article
Cite this article
Radin, M.D., Vinckeviciute, J., Seshadri, R. et al. Manganese oxidation as the origin of the anomalous capacity of Mn-containing Li-excess cathode materials. Nat Energy 4, 639–646 (2019). https://doi.org/10.1038/s41560-019-0439-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-019-0439-6
This article is cited by
-
Mn-based cathode materials for rechargeable batteries
Science China Chemistry (2024)
-
CHGNet as a pretrained universal neural network potential for charge-informed atomistic modelling
Nature Machine Intelligence (2023)
-
Inhibiting collective cation migration in Li-rich cathode materials as a strategy to mitigate voltage hysteresis
Nature Materials (2023)
-
Building Better Full Manganese-Based Cathode Materials for Next-Generation Lithium-Ion Batteries
Electrochemical Energy Reviews (2023)
-
Capturing dynamic ligand-to-metal charge transfer with a long-lived cationic intermediate for anionic redox
Nature Materials (2022)