Abstract
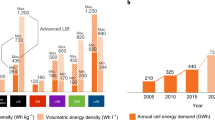
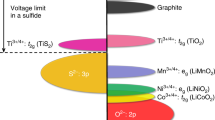
Enabling the reversible lithium metal electrode is essential for surpassing the energy content of today’s lithium-ion cells. Although lithium metal cells for niche applications have been developed already, efforts are underway to create rechargeable lithium metal batteries that can significantly advance vehicle electrification and grid energy storage. In this Perspective, we focus on three tasks to guide and further advance the reversible lithium metal electrode. First, we summarize the state of research and commercial efforts in terms of four key performance parameters, and identify additional performance parameters of interest. We then advocate for the use of limited lithium (≤30 μm) to ensure early identification of technical challenges associated with stable and dendrite-free cycling and a more rapid transition to commercially relevant designs. Finally, we provide a cost target and outline material costs and manufacturing methods that could allow lithium metal cells to reach 100 US$ kWh–1.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
02 August 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41560-022-01077-8
References
Pillot, C. Battery Market Development for Consumer Electronics, Automotive, and Industrial (2014).
Ryan, J. China is about to bury Elon Musk in batteries. Bloomberg (28 June 2017).
Schmidt, O., Hawkes, A., Gambhir, A. & Staffell, I. The future cost of electrical energy storage based on experience rates. Nat. Energy6, 17110 (2017).
Nykvist, B. & Nilsson, M. Rapidly falling costs of battery packs for electric vehicles. Nat. Clim. Change5, 329–332 (2015).
USABC Goals for Advanced Batteries for EVs — CY 2020 Commercialization (USABC, 2017).
Choi, J. W. & Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater.1, 16013 (2016).
Thackeray, M. M., Wolverton, C. & Isaacs, E. D. Electrical energy storage for transportation — approaching the limits of, and going beyond, lithium-ion batteries. Energ. Environ. Sci.5, 7854–7863 (2012).
Goodenough, J. B. & Park, K.-S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc.135, 1167–1176 (2013).
Christensen, J. et al. A critical review of Li/air batteries. J. Electrochem. Soc.159, R1–R30 (2012).
Albertus, P., Lohmann, T. & Christensen, J. in TheLi–AirBattery: Fundamentals (eds Imanishi, N., Luntz, A. C. & Bruce, P.) 291–310 (Springer, 2014).
McCloskey, B. D. Attainable gravimetric and volumetric energy density of Li–S and Li ion battery cells with solid separator-protected Li metal anodes. J. Phys. Chem. Lett.6, 4581–4588 (2015).
Gallagher, K. G. et al. Quantifying the promise of lithium–air batteries for electric vehicles. Energ. Environ. Sci.7, 1555–1563 (2014).
Mayer, S. W. & McKenzie, D. E. Lightweight secondary battery. US patent3(185), 590 (1965).
Whittingham, M. S. Electrical energy storage and intercalation chemistry. Science192, 1126–1127 (1976).
Tobias, C. W. & Jorne, J. Method of production of alkali metals and their alloys. US Patent US3791945 A (1974).
Hughes, M., Hampson, N. & Karunathilaka, S. A review of cells based on lithium negative electrodes (anodes). J. Power Sources12, 83–144 (1984).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energ. Environ. Sci.7, 513–537 (2014).
Tikekar, M. D., Choudhury, S., Tu, Z. & Archer, L. A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy1, 16114 (2016).
Takeda, Y., Yamamoto, O. & Imanishi, N. Lithium dendrite formation on a lithium metal anode from liquid, polymer and solid electrolytes. Electrochem84, 210–218 (2016).
Bates, J. B., Dudney, N. J., Neudecker, B., Ueda, A. & Evans, C. D. Thin-film lithium and lithium-ion batteries. Solid State Ionics135, 33–45 (2000).
Bates, J. B. et al. Fabrication and characterization of amorphous lithium electrolyte thin films and rechargeable thin-film batteries. J. Power Sources43, 103–110 (1993).
Hovington, P. et al. New lithium metal polymer solid state battery for an ultrahigh energy: nano C-LiFePO4 versus nano Li1.2V3O8. Nano Lett.15, 2671–2678 (2015).
Gross, J. & Eitouni, H. Drivers and technologies for Li-metal solid-state batteries. 16th Ann. Adv. Automot. Batt. Conf. (2016).
Monroe, C. & Newman, J. Dendrite growth in lithium/polymer systems. J. Electrochem. Soc.150, A1377–A1384 (2003).
Monroe, C. & Newman, J. The effect of interfacial deformation on electrodeposition kinetics. J. Electrochem. Soc.151, A880–A886 (2004).
Monroe, C. & Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc.152, A396–A404 (2005).
Tikekar, M. D., Archer, L. A. & Koch, D. L. Stabilizing electrodeposition in elastic solid electrolytes containing immobilized anions. Sci. Adv.2, e1600320 (2016).
Porz, L. et al. Mechanism of lithium metal penetration through inorganic solid electrolytes. Adv. Energ. Mater. https://doi.org/10.1002/aenm.201701003 (2017).
Aguesse, F. et al. Investigating the dendritic growth during full cell cycling of garnet electrolyte in direct contact with Li metal. ACS Appl. Mater. Interf.9, 3808–3816 (2017).
Cheng, E. J., Sharafi, A. & Sakamoto, J. Intergranular Li metal propagation through polycrystalline Li6.25Al0.25La3Zr2O12 ceramic electrolyte. Electrochim. Acta.223, 85–91 (2017).
Park, M. S. et al. A highly reversible lithium metal anode. Sci. Rep.4, 3815 (2014).
Tubandt, C. Handbuch der Experimentalphysik (Akad. Verlagsgesellschaft, Leipzig, 1932).
Reddy, T. Linden’s Handbook of Batteries (eds Reddy, T. & Linden, D.) (2011).
Aurbach, D., Zinigrad, E., Cohen, Y. & Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ionics148, 405–416 (2002).
Liu, Y. et al. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat. Commun.7, 10992 (2016).
Wang, C. et al. Conformal, nanoscale ZnO surface modification of garnet-based solid-state electrolyte for lithium metal anodes. Nano Lett.17, 565–571 (2016).
Albertus, P. Integration and Optimization of Novel Ion-Conducting Solids (IONICS) Funding Opportunity Announcement (2016); https://arpa-e-foa.energy.gov/FileContent.aspx?FileID=cfac9ce8-5a19-4623-b942-c3e65f3ccf77.
Hernandez-Maya, R., Rosas, O., Saunders, J. & Castaneda, H. Dynamic characterization of dendrite deposition and growth in Li-surface by electrochemical impedance spectroscopy. J. Electrochem. Soc.162, A687–A696 (2015).
Sharafi, A., Meyer, H. M., Nanda, J., Wolfenstine, J. & Sakamoto, J. Characterizing the Li–Li7La3Zr2O12 interface stability and kinetics as a function of temperature and current density. J. Power Sources302, 135–139 (2016).
Aurbach, D., Gofer, Y. & Langzam, J. The correlation between surface chemistry, surface morphology, and cycling efficiency of lithium electrodes in a few polar aprotic systems. J. Electrochem. Soc.136, 3198–3205 (1989).
Li, W. et al. The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth. Nat. Commun.6, 7436 (2015).
Qian, J. et al. High rate and stable cycling of lithium metal anode. Nat. Commun.6, 6362 (2015).
Tu, Z. et al. Nanoporous hybrid electrolytes for high‐energy batteries based on reactive metal anodes. Adv. Energ. Mater. 7, 1602367 (2017).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy1, 16030 (2016).
Neudecker, B., Dudney, N. & Bates, J. ‘Lithium‐free’ thin‐film battery with in situ plated Li anode. J. Electrochem. Soc.147, 517–523 (2000).
Murugan, R., Thangadurai, V. & Weppner, W. Fast lithium ion conduction in garnet‐type Li7La3Zr2O12. Angew. Chem. Int. Ed.46, 7778–7781 (2007).
Bron, P. et al. Li10SnP2S12: an affordable lithium superionic conductor. J. Am. Chem. Soc.135, 15694–15697 (2013).
Guo, J. et al. Cold sintering: a paradigm shift for processing and integration of ceramics. Angew. Chem.128, 11629–11633 (2016).
Guo, J. et al. Cold sintering process of composites: bridging the processing temperature gap of ceramic and polymer materials. Adv. Func. Mater.26, 7115–7121 (2016).
Acknowledgements
The authors thank N. Dudney, S. Visco, L. Archer, K. Xu, T. Holme and P. Frischmann for helpful discussions.
Author information
Authors and Affiliations
Contributions
P.A. conceived the figures, conducted the majority of the analysis and wrote most of the paper. S.B. and S.L. made significant contributions to the analysis and editing of the paper. A.N. contributed analysis and edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Dataset
Supplementary Table 1–4, Supplementary References.
Rights and permissions
About this article
Cite this article
Albertus, P., Babinec, S., Litzelman, S. et al. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat Energy 3, 16–21 (2018). https://doi.org/10.1038/s41560-017-0047-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-017-0047-2
This article is cited by
-
Hybridizing carbonate and ether at molecular scales for high-energy and high-safety lithium metal batteries
Nature Communications (2024)
-
Hierarchical Li electrochemistry using alloy-type anode for high-energy-density Li metal batteries
Nature Communications (2024)
-
Methylation enables the use of fluorine-free ether electrolytes in high-voltage lithium metal batteries
Nature Chemistry (2024)
-
Enhancing lithium-metal battery longevity through minimized coordinating diluent
Nature Energy (2024)
-
Unraveling electrolyte solvation architectures for high-performance lithium-ion batteries
Science China Technological Sciences (2024)