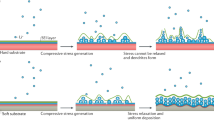
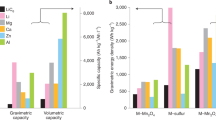
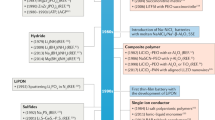
Abstract
The electrification of transport and the transition to renewable energy sources are driving demand for the versatile and efficient storage of electrical energy — principally batteries, which can store energy with high efficiency, in a variety of designs and sizes. Compared to conventional batteries that contain insertion anodes, next-generation rechargeable batteries with metal anodes can yield more favourable energy densities, thanks to their high specific capacities and low electrode potentials. In this Review, we cover recent progress in metal anodes for rechargeable batteries. We examine design concepts and application opportunities and highlight the differences between metal and insertion-type electrodes in interface (two-dimensional) and interphase (three-dimensional) chemistries. We conclude by analysing the available cell chemistries and architectures, focusing on the design strategies for sustainability, as well as discussing existing roadmaps for next-generation batteries.
Key points
-
Metal electrodes, which have large specific and volumetric capacities, can enable next-generation rechargeable batteries with high energy densities.
-
The charge and discharge processes for metal anodes (involving deposition and dissolution of metals) require reversible chemical reactions that constitute a major challenge.
-
Controlling the formation of interphases between the metal electrode and the electrolyte is key to achieving high reversibility and long cycle life, as well as fast-charging rates.
-
There is no universal concept for the design of advanced electrolytes or a single strategy for the control of interface chemistry, meaning that an improved understanding of what redox processes occur is needed.
-
The transition from developing cell chemistries to designing the complete metal-anode battery is critical, because the manufacture of thin metal foils and the assembly of prototype cells are not straightforward.
-
For real applications, the fast-charging requirements (related to electron and ion transport) do not at present favour alternatives to Li- or Na-anode systems; batteries with anodes made using low-cost and abundant materials (Al, Mg, Ca, Na) are suitable for stationary storage systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Winter, M. & Brodd, R. J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 104, 4245–4270 (2004). The basic operating principles of batteries and definitions of terms relevant for batteries are presented, as well as the thermodynamic and kinetic origins of electrochemical reactions.
Adenusi, H., Chass, G. A., Passerini, S., Tian, K. V. & Chen, G. Lithium batteries and the solid electrolyte interphase (SEI) — progress and outlook. Adv. Energy Mater. 13, 2203307 (2023).
Xiang, J. et al. Alkali-metal anodes: from lab to market. Joule 3, 2334–2363 (2019).
Zhang, X. et al. Challenges and opportunities for multivalent metal anodes in rechargeable batteries. Adv. Funct. Mater. 30, 2004187 (2020). In this paper, the fundamental mechanisms and key issues of multivalent metal anodes are discussed and strategies towards the development of future cell chemistries are presented.
Maddegalla, A. et al. AZ31 magnesium alloy foils as thin anodes for rechargeable magnesium batteries. ChemSusChem 14, 4690–4696 (2021).
Ran, Q. et al. Aluminum–copper alloy anode materials for high-energy aqueous aluminum batteries. Nat. Commun. 13, 576 (2022).
Xu, C., Li, B., Du, H. & Kang, F. Energetic zinc ion chemistry: the rechargeable zinc ion battery. Angew. Chem. Int. Edn Engl. 51, 933–935 (2012).
Tchitchekova, D. S. et al. Electrochemical intercalation of calcium and magnesium in TiS2: fundamental studies related to multivalent battery applications. Chem. Mater. 30, 847–856 (2018).
Winter, M. The solid electrolyte interphase — the most important and the least understood solid electrolyte in rechargeable Li batteries. Z. Phys. Chem. 223, 1395–1406 (2009).
US Geological survey mineral commodity summaries 2023. USGS https://doi.org/10.3133/mcs2023 (2023).
Duffner, F. et al. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 6, 123–134 (2021). This review analyses post-lithium ion battery production and market fabrication, including solid-state lithium- and sodium-based batteries.
Resources and energy quarterly: June 2023. Australian Government. Department of Industry, Science and Resources https://www.industry.gov.au/publications/resources-and-energy-quarterly-june-2023 (2023).
Liu, J. et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019). In this perspective, strategies for long-cycling and high-energy Li-metal-based cells are introduced and key factors that affect cell performance are identified.
Arroyo-de Dompablo, M. E., Ponrouch, A., Johansson, P. & Palacín, M. R. Achievements, challenges, and prospects of calcium batteries. Chem. Rev. 120, 6331–6357 (2020).
Zhao-Karger, Z. et al. Calcium–tin alloys as anodes for rechargeable non-aqueous calcium-ion batteries at room temperature. Nat. Commun. 13, 3849 (2022).
Blanc, L. E., Kundu, D. & Nazar, L. F. Scientific challenges for the implementation of Zn-ion batteries. Joule 4, 771–799 (2020). A broad overview of key challenges and strategies for the development of Zn-based batteries is provided, including recent progress in materials research.
Sun, W. et al. A rechargeable zinc — air battery based on zinc peroxide chemistry. Science 371, 46–51 (2021).
Ahn, H., Kim, D., Lee, M. & Nam, K. W. Challenges and possibilities for aqueous battery systems. Commun. Mater. 4, 37 (2023).
Yang, H. et al. The rechargeable aluminum battery: opportunities and challenges. Angew. Chem. Int. Edn 58, 11978–11996 (2019). This review describes rechargeable aluminum batteries and illustrates their opportunities and challenges.
Liu, W., Liu, P. & Mitlin, D. Review of emerging concepts in SEI analysis and artificial SEI membranes for lithium, sodium, and potassium metal battery anodes. Adv. Energy Mater. 10, 2002297 (2020).
Peled, E. & Menkin, S. Review — SEI: past, present and future. J. Electrochem. Soc. 164, A1703–A1719 (2017).
Lennartz, P. et al. Practical considerations for enabling Li|polymer electrolyte batteries. Joule 7, 1471–1495 (2023).
Peled, E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems — the solid electrolyte interphase model. J. Electrochem. Soc. 126, 2047–2051 (1979).
Winter, M. & Besenhard, J. O. Wiederaufladbare batterien. Chem. Uns. Zeit 33, 252–266 (1999).
Gallus, D. R., Wagner, R., Wiemers-Meyer, S., Winter, M. & Cekic-Laskovic, I. New insights into the structure–property relationship of high-voltage electrolyte components for lithium-ion batteries using the pKa value. Electrochim. Acta 184, 410–416 (2015).
Meister, P. et al. Best practice: performance and cost evaluation of lithium ion battery active materials with special emphasis on energy efficiency. Chem. Mater. 28, 7203–7217 (2016).
Kühn, S. P., Edström, K., Winter, M. & Cekic‐Laskovic, I. Face to face at the cathode electrolyte interphase: from interface features to interphase formation and dynamics. Adv. Mater. Interf. 9, 2102078 (2022).
Betz, J. et al. Cross talk between transition metal cathode and Li metal anode: unraveling its influence on the deposition/dissolution behavior and morphology of lithium. Adv. Energy Mater. 9, 1900574 (2019).
Takenaka, N., Bouibes, A., Yamada, Y., Nagaoka, M. & Yamada, A. Frontiers in theoretical analysis of solid electrolyte interphase formation mechanism. Adv. Mater. 33, 2100574 (2021).
Zhang, Z. et al. Capturing the swelling of solid–electrolyte interphase in lithium metal batteries. Science 375, 66–70 (2022).
Popovic, J. The importance of electrode interfaces and interphases for rechargeable metal batteries. Nat. Commun. 12, 6240 (2021).
Borzutzki, K., Nair, J. R., Winter, M. & Brunklaus, G. Does cell polarization matter in single-ion conducting electrolytes? ACS Appl. Mater. Interf. 14, 5211–5222 (2022).
Lee, B., Paek, E., Mitlin, D. & Lee, S. W. Sodium metal anodes: emerging solutions to dendrite growth. Chem. Rev. 119, 5416–5460 (2019).
Wang, J. et al. Improving cyclability of Li metal batteries at elevated temperatures and its origin revealed by cryo-electron microscopy. Nat. Energy 4, 664–670 (2019).
Zachman, M. J., Tu, Z., Choudhury, S., Archer, L. A. & Kourkoutis, L. F. Cryo-STEM mapping of solid–liquid interfaces and dendrites in lithium-metal batteries. Nature 560, 345–349 (2018).
Hope, M. A. et al. Selective NMR observation of the SEI–metal interface by dynamic nuclear polarisation from lithium metal. Nat. Commun. 11, 2224 (2020).
Blázquez, J. A. et al. A practical perspective on the potential of rechargeable Mg batteries. Energy Environ. Sci. 16, 1964–1981 (2023). Concepts for Mg-based batteries are critically reviewed, their potential applications are discussed and a non-aqueous multilayer magnesium-based multilayer prototype cell is pioneered.
Zhang, H. et al. Electrolyte additives for lithium metal anodes and rechargeable lithium metal batteries: progress and perspectives. Angew. Chem. Int. Edn Engl. 57, 15002–15027 (2018).
Janek, J. & Zeier, W. G. Challenges in speeding up solid-state battery development. Nat. Energy 8, 230–240 (2023).
Goodenough, J. B. & Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603 (2010).
Wang, H. et al. Liquid electrolyte: the nexus of practical lithium metal batteries. Joule 6, 588–616 (2022).
Cekic–Laskovic, I. et al. Synergistic effect of blended components in nonaqueous electrolytes for lithium ion batteries. Top. Curr. Chem. 375, 37 (2017).
Yu, Z. et al. Rational solvent molecule tuning for high-performance lithium metal battery electrolytes. Nat. Energy 7, 94–106 (2022).
Chae, O. B. & Lucht, B. L. Interfacial issues and modification of solid electrolyte interphase for Li metal anode in liquid and solid electrolytes. Adv. Energy Mater. 13, 2203791 (2023). In this perspective, the role of the electrode|electrolyte interphases for the reversibility of Li-metal anodes is emphasized, including design strategies to reinforce the naturally occurring SEI layer.
Li, S. et al. Functional polymers for lithium metal batteries. Prog. Polym. Sci. 122, 101453 (2021).
Zhou, L. et al. High areal capacity, long cycle life 4 V ceramic all-solid-state Li-ion batteries enabled by chloride solid electrolytes. Nat. Energy 7, 83–93 (2022).
Zhang, K. et al. 8.5-µm‐thick flexible–rigid hybrid solid–electrolyte/lithium integration for air‐stable and interface‐compatible all‐solid‐state lithium metal batteries. Adv. Energy Mater. 12, 2200368 (2022).
Chen, Y.-H. et al. Towards all-solid-state polymer batteries: going beyond PEO with hybrid concepts. Adv. Funct. Mater. 33, 2300501 (2023).
Yin, Y. et al. Fire-extinguishing, recyclable liquefied gas electrolytes for temperature-resilient lithium-metal batteries. Nat. Energy 7, 548–559 (2022).
Usiskin, R. et al. Fundamentals, status and promise of sodium-based batteries. Nat. Rev. Mater. 6, 1020–1035 (2021). The fundamental principles of Na-based batteries are compared to those of Li-based batteries, and prototypes of Na cells are discussed.
Mandai, T. et al. Toward improved anodic stability of ether-based electrolytes for rechargeable magnesium batteries. J. Phys. Chem. C 127, 10419–10433 (2023).
Okuo, T., Mandai, T., Masunaga, H., Ohta, N. & Matsumoto, H. Magnesiated Nafion-based gel electrolytes: structural and electrochemical characterization. J. Phys. Chem. C https://doi.org/10.1021/acs.jpcc.3c02924 (2023).
Mandai, T., Naya, H. & Masu, H. Comparative studies on [B(HFIP) 4]-based electrolytes with mono- and divalent cations. J. Phys. Chem. C 127, 7987–7997 (2023).
Martin, P., Årén, F. & Johansson, P. (Localized) highly concentrated electrolytes for calcium batteries. Batt. Supercaps 6, e202300003 (2023).
Cao, X., Jia, H., Xu, W. & Zhang, J.-G. Review — localized high-concentration electrolytes for lithium batteries. J. Electrochem. Soc. 168, 010522 (2021).
Ng, K. L., Amrithraj, B. & Azimi, G. Nonaqueous rechargeable aluminum batteries. Joule 6, 134–170 (2022).
Xiang, J. et al. Building practical high‐voltage cathode materials for lithium‐ion batteries. Adv. Mater. 34, 2200912 (2022).
Ramasubramanian, B. et al. Recent development in carbon-LiFePO4 cathodes for lithium-ion batteries: a mini review. Batteries 8, 133 (2022).
Zhao, C. et al. A high-energy and long-cycling lithium–sulfur pouch cell via a macroporous catalytic cathode with double-end binding sites. Nat. Nanotechnol. 16, 166–173 (2021).
Ou, X. et al. Enabling high energy lithium metal batteries via single-crystal Ni-rich cathode material co-doping strategy. Nat. Commun. 13, 2319 (2022).
Xu, Z. & Wang, J. Toward emerging sodium‐based energy storage technologies: from performance to sustainability. Adv. Energy Mater. 12, 2201692 (2022).
Tang, X. et al. A universal strategy towards high-energy aqueous multivalent-ion batteries. Nat. Commun. 12, 2857 (2021). Concepts for aqueous batteries based on Ca, Mg and Al are reviewed, demonstrating the redox chemistry for aqueous prototype cells.
Aurbach, D. et al. Prototype systems for rechargeable magnesium batteries. Nature 407, 724–727 (2000).
Kwon, B. J. et al. High voltage Mg-ion battery cathode via a solid solution Cr–Mn spinel oxide. Chem. Mater. 32, 6577–6587 (2020).
Kisu, K., Mohtadi, R. & Orimo, S. Calcium metal batteries with long cycle life using a hydride‐based electrolyte and copper sulfide electrode. Adv. Sci. 10, 2301178 (2023).
Kim, S. et al. Investigation of rechargeable calcium metal–selenium batteries enabled by borate-based electrolytes. Chem. Mater. 35, 2363–2370 (2023).
Richard Prabakar, S. J. et al. Ultra‐high capacity and cyclability of β‐phase Ca0.14V2O5 as a promising cathode in calcium‐ion batteries. Adv. Funct. Mater. 33, 2301399 (2023).
Zhang, X. et al. Towards a stable layered vanadium oxide cathode for high‐capacity calcium batteries. Small 18, 2107174 (2022).
Placke, T., Kloepsch, R., Dühnen, S. & Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: the odyssey for high energy density. J. Solid State Electrochem. 21, 1939–1964 (2017).
Mandai, T. & Somekawa, H. Ultrathin magnesium metal anode — an essential component for high‐energy‐density magnesium battery materialization. Batt. Supercaps 5, https://doi.org/10.1002/batt.202200153 (2022).
Huang, C.-J. et al. Decoupling the origins of irreversible coulombic efficiency in anode-free lithium metal batteries. Nat. Commun. 12, 1452 (2021).
Wang, C. et al. High dielectric barium titanate porous scaffold for efficient Li metal cycling in anode-free cells. Nat. Commun. 12, 6536 (2021).
Merso, S. K. et al. An in-situ formed bifunctional layer for suppressing Li dendrite growth and stabilizing the solid electrolyte interphase layer of anode free lithium metal batteries. J. Energy Storage 56, 105955 (2022).
Fedeli, E. et al. Towards advanced lithium metal solid-state batteries: durable and safe multilayer pouch cell enabled by a nanocomposite solid electrolyte. Solid State Ion. 392, 116148 (2023).
Edström, K. et al. BATTERY 2030+ Roadmap. Inventing the sustainable batteries of the future. Research needs and future actions; https://battery2030.eu/wp-content/uploads/2022/07/BATTERY-2030-Roadmap_Revision_FINAL.pdf (2022).
Niu, C. et al. Balancing interfacial reactions to achieve long cycle life in high-energy lithium metal batteries. Nat. Energy 6, 723–732 (2021).
Frenck, L. et al. Failure mechanisms at the interfaces between lithium metal electrodes and a single-ion conducting polymer gel electrolyte. ACS Appl. Mater. Interf. 14, 53893–53903 (2022).
Yang, C., Sunderlin, N., Wang, W., Churchill, C. & Keyser, M. Compressible battery foams to prevent cascading thermal runaway in Li-ion pouch batteries. J. Power Sources 541, 231666 (2022).
Jia, Y. et al. Data‐driven safety risk prediction of lithium‐ion battery. Adv. Energy Mater. 11, 2003868 (2021).
Greenwood, M. et al. The Battery Component Readiness Level (BC-RL) framework: a technology-specific development framework. J. Power Sources Adv 14, 100089 (2022).
Li, J. et al. Polymers in lithium‐ion and lithium metal batteries. Adv. Energy Mater. 11, 2003239 (2021).
Borzutzki, K., Thienenkamp, J., Diehl, M., Winter, M. & Brunklaus, G. Fluorinated polysulfonamide based single ion conducting room temperature applicable gel-type polymer electrolytes for lithium ion batteries. J. Mater. Chem. Mater. 7, 188–201 (2019).
Huo, H. & Janek, J. Silicon as emerging anode in solid-state batteries. ACS Energy Lett 7, 4005–4016 (2022).
Arnold, S., Wang, L. & Presser, V. Dual‐use of seawater batteries for energy storage and water desalination. Small 18, 2107913 (2022).
Kim, Y. et al. Anode-less seawater batteries with a Na-ion conducting solid-polymer electrolyte for power to metal and metal to power energy storage. Energy Environ. Sci. 15, 2610–2618 (2022).
Kim, Y. et al. Large-scale stationary energy storage: seawater batteries with high rate and reversible performance. Energy Storage Mater. 16, 56–64 (2019).
Neumann, J. et al. Recycling of lithium‐ion batteries — current state of the art, circular economy, and next generation recycling. Adv. Energy Mater. 12, 2102917 (2022).
Doose, S., Mayer, J. K., Michalowski, P. & Kwade, A. Challenges in ecofriendly battery recycling and closed material cycles: a perspective on future lithium battery generations. Metals 11, 291 (2021).
European Commission. Proposal for a Regulation of the European Parliament and of the Council concerning batteries and waste batteries, repealing Directive 2006/66/EC and amending Regulation (EU) no 2019/1020; https://eur-lex.europa.eu/resource.html?uri=cellar:4b5d88a6-3ad8-11eb-b27b-01aa75ed71a1.0001.02/DOC_1&format=PDF (2020).
Börner, M. F. et al. Challenges of second-life concepts for retired electric vehicle batteries. Cell Rep. Phys. Sci. 3, 101095 (2022).
Zeng, A. et al. Battery technology and recycling alone will not save the electric mobility transition from future cobalt shortages. Nat. Commun. 13, 1341 (2022).
Baars, J., Domenech, T., Bleischwitz, R., Melin, H. E. & Heidrich, O. Circular economy strategies for electric vehicle batteries reduce reliance on raw materials. Nat. Sustain. 4, 71–79 (2020).
Wang, T. et al. A hetero‐layered, mechanically reinforced, ultra‐lightweight composite polymer electrolyte for wide‐temperature‐range, solid‐state sodium batteries. Adv. Funct. Mater. 33, 2215117 (2023).
Wang, D. et al. Realizing high-capacity all-solid-state lithium-sulfur batteries using a low-density inorganic solid-state electrolyte. Nat. Commun. 14, 1895 (2023).
Li, C. et al. Highly reversible Zn anode with a practical areal capacity enabled by a sustainable electrolyte and superacid interfacial chemistry. Joule 6, 1103–1120 (2022).
Acknowledgements
The authors gratefully acknowledge generous support by the German Federal Ministry of Education and Research (BMBF) within the grants FB2-POLY (13XP0429A), FB2-Hybrid (13XP0428A) and LISI-2 (13XP0509A).
Author information
Authors and Affiliations
Contributions
G.B. and P.L. contributed equally to this paper. All the authors contributed substantially to discussion of the content. G.B. and P.L. wrote the article. All the authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors have no competing interests to declare.
Peer review
Peer review information
Nature Reviews Electrical Engineering thanks Xuefeng Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Bluecar: https://www.bluesystems.ai/technology/bluecar/
Bolloré: https://www.bollore.com/en/electricity-storage-and-solutions/
LiNa Energy: https://www.lina.energy/technology/
Lithion: https://www.lithiontechnologies.com/lithium-ion-battery-recycling
NAS Battery: https://www.basf.com/global/en/who-we-are/organization/group-companies/BASF-Stationary-Energy-Storage-GmbH.html
Quantumscape: https://www.quantumscape.com/
SES: https://ses.ai/
Tiamat: http://www.tiamat-energy.com/
TRL: https://www.nasa.gov/directorates/heo/scan/engineering/technology/technology_readiness_level
Umicore: https://pmr.umicore.com/en/about-us/
US Advanced Battery Consortium: https://uscar.org/usabc/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brunklaus, G., Lennartz, P. & Winter, M. Metal electrodes for next-generation rechargeable batteries. Nat Rev Electr Eng 1, 79–92 (2024). https://doi.org/10.1038/s44287-023-00006-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44287-023-00006-5