Abstract
The conversion of natural habitats to farmland is a major cause of biodiversity loss and poses the greatest extinction risk to birds worldwide. Tropical raptors are of particular concern, being relatively slow-breeding apex predators and scavengers, whose disappearance can trigger extensive cascading effects. Many of Africa’s raptors are at considerable risk from habitat conversion, prey-base depletion and persecution, driven principally by human population expansion. Here we describe multiregional trends among 42 African raptor species, 88% of which have declined over a ca. 20–40-yr period, with 69% exceeding the International Union for Conservation of Nature criteria classifying species at risk of extinction. Large raptors had experienced significantly steeper declines than smaller species, and this disparity was more pronounced on unprotected land. Declines were greater in West Africa than elsewhere, and more than twice as severe outside of protected areas (PAs) than within. Worryingly, species suffering the steepest declines had become significantly more dependent on PAs, demonstrating the importance of expanding conservation areas to cover 30% of land by 2030—a key target agreed at the UN Convention on Biological Diversity COP15. Our findings also highlight the significance of a recent African-led proposal to strengthen PA management—initiatives considered fundamental to safeguarding global biodiversity, ecosystem functioning and climate resilience.
Similar content being viewed by others
Main
The conversion of wooded habitats to agricultural land is more damaging to biodiversity than any other human activity1,2,3,4 and poses the greatest extinction risk to birds worldwide2,3. Tropical raptors are especially vulnerable, being particularly slow-breeding5,6 and subject to a wide range of threats linked to rapid human population growth, farmland expansion7,8,9,10 and habitat fragmentation11. While resident tropical raptors thus have great potential as a model system for investigating land-use change impacts, trends in their abundance have been little studied so far, reflecting the paucity of suitable long-term survey data and a limited capacity for conservation research in most developing countries12. Here we present a multiregional assessment of trends among many of Africa’s widespread, diurnal raptor species, and compare rates of change in their abundance within protected and unprotected areas.
Africa is exceptionally important for global raptor conservation, supporting high numbers of threatened species13. Over the past ca. 60 yr, however, the continent’s human population has expanded rapidly10, driving widespread land conversion and habitat degradation, and creating areas where cumulative human impacts on threatened raptors are especially acute9. Sub-Saharan Africa lost almost 5 million ha of forest and non-forest natural vegetation per annum during 1975–2000 alone14 and now experiences the most severe rate of land degradation in the world15. With its human population projected to double by 2058, demands for grazing, arable land and energy are expected to rise substantially10,16. These trends will amplify existing pressures on Africa’s protected areas (PAs), which currently account for just 14% of its land and inland waters17. Although many PAs are considered to be failing or deteriorating18,19, well-managed sites form a critical refuge for the continent’s declining raptor populations20,21,22,23.
Additional threats to Africa’s avian apex predators, meso-predators and scavengers include prey-base depletion13, persecution (shooting, trapping, poisoning)24, unintentional poisoning25, electrocution/collision with energy infrastructure26,27,28,29 and killing for food and belief-based uses30,31,32. These pressures are typically more acute within unprotected land and have probably impacted larger raptor species more severely, reflecting global patterns of extinction risk among terrestrial mammalian predators33. Importantly, the loss and depletion of predator populations not only affects the species concerned, but can also trigger extensive cascading effects among their prey populations, disrupting ecosystem functioning9,34,35,36,37. Ecosystem services provided by raptors include the rapid removal of carcasses, potentially limiting the transmission of zoonotic diseases to human populations37,38,39.
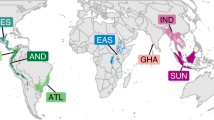
Despite these pressures, and the keystone role played by many raptor species, attempts to measure trends in their abundance have been hindered by the absence of systematic, pan-African bird monitoring programmes, generating robust, long-term trend data for this species group. Here, based on repeated raptor road transect surveys undertaken in four African regions, we examine changes in encounter rates (individuals recorded per 100 km) among 42 species dependent mainly on savanna habitats. To determine rates of change, we combined published and unpublished road transect data from surveys conducted during 1969–1995 and 2000–2020 in West Africa (Burkina Faso, Niger and Mali)40, Central Africa (northern Cameroon)41, East Africa (Kenya)42 and southern Africa (northern Botswana)20,43 (Fig. 1, Supplementary Table 1 and Extended Data Fig. 1). Pooling these data has provided unprecedented insights into trends in the abundance of Africa’s savanna raptors, enabling us to identify species whose composite decline estimates exceed the limits defining their current International Union for Conservation of Nature (IUCN) threat status. We also determine the extent to which decline rates differed between selected PA categories and unprotected land, and investigate potential links between abundance change, body size and protected area dependency.
Road transects were conducted in West Africa, northern Cameroon and Kenya in 1969–1977 and 2000–2020, and in northern Botswana in 1991–1995 and 2015–2016. Here, orange shading indicates parts of the global range of bateleur Terathopius ecaudatus that lie within road transect countries and overlap with areas where climatic conditions match those of the routes surveyed in that country. Grey shading indicates the rest of the species’ range within surveyed and unsurveyed countries alike. Bar charts show percentage change in the number of individuals encountered per 100 km within protected and unprotected areas (PAs and UPAs), projected over three generation lengths; 44 yr in this instance. The species’ trajectory within its South African range (mauve) was derived from SABAP2 reporting rates during 2008–2021. Photograph: © André Botha.
Results
We found strong evidence of widespread declines among African raptor species spanning up to 40 yr (Table 1). Overall, 37 (88%) of the 42 species examined had declined, 29 (69%) by at least 30% over three generation lengths—a criterion used by IUCN to identify species at risk of global extinction44. Of 27 species surveyed in multiple regions, 24 (89%) had exceeded this decline threshold (Fig. 2), 13 of which are currently classified as Least Concern45. While 7 of these 13 species have extensive global ranges outside of Africa, where trends may differ from those reported here, the remaining 6 are African endemics or near-endemics (Fig. 2).
Fifteen species were surveyed adequately in single regions only (grey bars). The remaining 27 were each surveyed in two regions (lighter green bars) or 3–4 regions (dark green). Bar length shows a given species’ median rate of change in abundance, estimated under two scenarios, in which average encounter rates in unsurveyed PAs were assumed to have been the same as in surveyed PAs, or the same as in UPAs (Methods). Points overlaid on bars show individual change estimates, where the sample size (n = 4, 16, 64 or 256) reflects the number of studies in which the species was surveyed (1, 2, 3 or 4 studies); error bars show the Q1–Q3 range. Twenty-nine species had declined at rates exceeding the IUCN Vulnerable threshold; 24 had exceeded the limits defining their current threat category. Fifteen of these are African endemics or near-endemics, 6 of which (illustrated) were surveyed in multiple regions and are currently listed as Least Concern. Silhouettes drawn from photographs: © André Botha.
Large raptors showed more rapid declines
The annual rate of change in encounter rates within the four regions combined was inversely related to body mass, with larger species showing significantly steeper declines (effect size = –0.016 × sqrt(mass, kg), R2 = 0.109, P = 0.0185; model 1 in Extended Data Table 1). This relationship was amplified when projected over three generation lengths (effect size = –0.351 × sqrt(mass, kg), R2 = 0.254, P = 0.0004; model 2 in Extended Data Table 1), since generation length itself is positively correlated with body mass (effect size = 2.555 × log(mass, g), R2 = 0.830, P < 0.0001; model 3 in Extended Data Table 1). We note, however, that this pattern was strongly influenced by the 10 heaviest species, all of which had declined at rates exceeding 60% over three generation lengths (Fig. 3). Thus, larger, apex raptors and scavengers had declined more rapidly per annum than smaller species, and since larger species tend to live longer, this relationship was more pronounced when projected over three generation lengths.
Each point represents one species (n = 42), grouped taxonomically as in Supplementary Table 2. Circle size indicates the number of regions in which the species was surveyed (n = 1–4). Rates of change were more variable among small–medium raptors than among large species (≥1,300 g; dashed line). Most large raptors had declined by at least 80% over three generation lengths, partly reflecting the positive relationship between body mass and longevity.
Rates of change varied between regions
Raptor population decline rates were significantly more severe in West Africa than elsewhere. In Central, East and southern Africa, there was no significant regional variation in encounter rate trends (χ22 = 0.2113, P = 0.8997; model 4 in Extended Data Table 1), and the median annual rate of change was −2.3%. In West Africa, encounter rates for the same species had declined more than twice as rapidly, at a median of −5.4% per annum (χ21 = 13.288, P = 0.0003; model 5 in Extended Data Table 1).
To extend our geographical coverage within southern Africa, we determined the direction of change in atlas reporting rates in South Africa for 30 of the 42 species, using data from the Southern African Bird Atlas Project (SABAP2) spanning 2008–202146. Reporting rates for 15 species had changed significantly (Bonferroni correction applied), of which 9 (60%) had suffered declines. Ten of the 15 species showed the same direction of change in South Africa as was evident from road transect surveys elsewhere (Table 1; concordance no greater than chance: χ21 = 1.666, P = 0.1967).
Decline rates derived from road transect surveys showed a negative but non-significant association with migratory status, after controlling for body-mass effects. The mean annual rate of decline among 14 species that are either migratory or have both migratory and sedentary populations in Africa was 52% higher than among 28 wholly sedentary species (effect size = 0.015, R2 = 0.170, P = 0.0989; model 6 in Extended Data Table 1).
Raptor declines were less severe within PAs than elsewhere
In each region, the median annual decline rate was greater in unprotected areas (UPAs) than within the protected area types assessed here (Fig. 4a), significantly so in the case of West Africa (Wilcoxon signed-ranks test: V = 349, P = 0.0005; model 7 in Extended Data Table 1) and Kenya (V = 229, P = 0.0004; model 8 in Extended Data Table 1). Overall, 33 (79%) of the 42 species had declined more rapidly in UPAs, as had 24 (89%) of the 27 species surveyed in multiple regions. The median annual rate of decline among the 42 species assessed was 2.3 times higher in UPAs (−2.66%, quartiles: −1.74% to −5.25%) than in PAs (−1.15%, quartiles: +0.06% to −2.18%) (V = 792, P < 0.0001; model 9 in Extended Data Table 1). Similarly, the median rate of decline over three generation lengths was 2.5 times higher in UPAs (−48%, quartiles: −27% to −78%) than in PAs (−19%, quartiles: +1% to −49%) (V = 765, P < 0.0001; model 10 in Extended Data Table 1). Thus, while many species had declined in both protected and unprotected areas, annual rates of decline were more than twice as high in the latter.
Results from protected and unprotected areas are shown in orange and grey, respectively. a, In all four road transect studies, median annual decline rates in UPAs exceeded those within the PAs assessed, significantly so in West Africa and Kenya. Boxplots show the median, first and third quartiles of the change in abundance within protected and unprotected areas in each of the four regions. Whiskers extend to ±1.5× the interquartile range. Each point represents one species; n = 28 (West Africa), 15 (N. Cameroon), 22 (Kenya) and 25 (N. Botswana). b, The effects of site protection were more pronounced among large (≥1,300 g) than among small–medium raptors. Median decline rates in PAs vs UPAs differed by 30 percentage points among large raptors and by 18 percentage points among small–medium species. Boxplots show the median, first and third quartiles of the rate of change in abundance of large vs small–medium raptor species, inside vs outside of protected areas. Whiskers extend to ±1.5× the interquartile range. Each point represents one species; n = 15 large, 27 small–medium species. c, Modelled relationship between the rate of change in abundance, body mass and protected area status (PAs vs UPAs). Notably, declines over three generation lengths exceeded the IUCN Vulnerable threshold (−30%, blue line) for the bulk of species in UPAs and for most large raptors within the PA types assessed. Fitted lines and shading indicate modelled change rates ±1 s.e.m. (model 13 in Extended Data Table 1).
When PA effects were controlled for, large raptors (>1,300 g; Supplementary Table 2) continued to show steeper annual declines than smaller species (χ21 = 5.781, P = 0.0162; model 11 in Extended Data Table 1). Projected over three generation lengths, decline rates of large raptors were substantially higher than those of smaller species, within PAs (median change: −50.5% vs −13.5%) as well as UPAs (−80.7% vs −31.9%) (χ21 = 20.942, P < 0.0001; model 12 in Extended Data Table 1). The influence of body mass on decline rate was thus greater on unprotected land (a difference of 49 percentage points) than on protected land (37 percentage points) (χ21 = 10.491, P = 0.0012; model 12 in Extended Data Table 1) (Fig. 4b). Notably, even within PAs, decline rates of most large species had exceeded the IUCN Vulnerable threshold (−30% over three generation lengths) (Fig. 4c; model 13 in Extended Data Table 1). Indeed, 17 (40%) of the 42 species had declined within PAs at rates exceeding the Vulnerable, Endangered or Critically Endangered threshold, compared with 27 species (64%) in UPAs. Thus, although population declines within the PA types assessed were lower than elsewhere, particularly for large raptor species, in some cases they still exceeded IUCN thresholds classifying species at risk of extinction.
Reliance on protected areas had increased significantly
To further examine the role of protected areas as potential refugia for raptor populations, we measured the disparity between each species’ encounter rates within the PA types we assessed and in UPAs, as an index of its dependence on the former. A positive index value indicated a higher encounter rate within PAs, and values potentially ranged from +1.0 (recorded only in PAs) to −1.0 (recorded only in UPAs). In each survey period, large raptors were significantly more dependent on PAs than were smaller species (χ21 = 4.461, P = 0.0346, n = 84; model 14 in Extended Data Table 1). Between the two periods, 29 (69%) of the 42 species had become more dependent on PAs, with the median dependency score rising from 0.56 to 0.83 for large raptors and from 0.15 to 0.44 for smaller species (χ21 = 12.151, P = 0.0005, n = 84; model 14 in Extended Data Table 1) (Fig. 5a).
Index values potentially ranged from +1.0 (recorded only within PAs) to −1.0 (recorded only in UPAs). a, Boxplot showing PA dependency scores in relation to survey period (green, 1969–1995; blue, 2000–2020) and body size class. In each period, large raptors were significantly more dependent on PAs than small–medium species. Notably, for species in both size classes, PA dependency increased significantly between 1969–1995 and 2000–2020. Boxplots show the median, first and third quartiles. Whiskers extend to ±1.5× the interquartile range. Each point represents one species; n = 15 large, 27 small–medium. b, Scatterplot showing annual change in abundance vs change in dependency on protected areas.The extent to which a species’ dependence on PAs changed between the two periods was significantly correlated with change in abundance. Species whose encounter rates had declined sharply had become more dependent on PAs than those showing a moderate decline or increase. Each point represents one species (n = 42); the fitted line and shading show modelled change rates ±1 s.e.m. (model 16 in Extended Data Table 1).
The widening disparity between raptor abundance levels in PAs and UPAs was driven by differences in decline rates. While encounter rates in UPAs fell by a median of 54% (Wilcoxon signed-rank test: V = 849.0, P < 0.0001; model 15 in Extended Data Table 1), in PAs they fell by a median of 19% (V = 633.5, P < 0.0232; model 15 in Extended Data Table 1). Thus, raptors had become less abundant both within PAs and UPAs, indicating that the growing disparity arose more from a rapid deterioration in conditions outside of protected areas than from improving or stable conditions within.
Rapidly declining species had become more PA-dependent
Interestingly, the rate of change in abundance was correlated with change in a species’ dependence on protected areas (effect size = −0.033, R2 = 0.189, P = 0.0024; model 16 in Extended Data Table 1). However, since both measures were derived from encounter rate values, we caution that the nature of this relationship may have been influenced by a high level of endogeneity within the model. Nevertheless, our findings indicate that species suffering the sharpest drop in abundance had become more dependent on protected areas than those showing little or no change (Fig. 5b).
Discussion
Over periods of ca. 20–40 yr, many of the 42 African raptor species examined had endured a double jeopardy – of precipitous population declines coupled with an increasing reliance on protected areas. While declines on a similar geographic scale have been reported previously for African vultures47, this study encompasses a much larger, more ecologically diverse group of savanna predators and scavengers, whose trajectories are more likely to reflect the broad range of pressures now facing African raptor populations.
Our trend analyses leveraged published road transect studies, whose key findings were in broad agreement with those of single-species studies employing more tailored survey methods22,48,49,50. They indicate that as a group, Africa’s diurnal raptors are facing an extinction crisis, with more than two-thirds of the species examined potentially qualifying as globally threatened. Notably, 13 of those surveyed in multiple regions are currently listed by IUCN as Least Concern (Table 1). A further 6 species recognized as globally threatened (secretarybird Sagittarius serpentarius, lappet-faced vulture Torgos tracheliotos, bateleur, tawny eagle Aquila rapax, steppe eagle A. nipalensis and martial eagle Polemaetus bellicosus) had declined more rapidly than the threshold rates used to define their current threat status. Our findings thus highlight the need to reassess their status at the earliest opportunity.
In contrast, our decline rate for hooded vulture (−67% over three generations) was much lower than that estimated in 201647 (−83%) and on which the species’ current threat status (Critically Endangered) was initially based. This follows a recent review51 in which the species’ generation length estimate was substantially shortened, reducing the apparent scale of its decline over three generation lengths. Hooded vulture remains Critically Endangered, however, following a surge in demand for vulture body parts in West Africa, its stronghold region32,45. Three additional species showing steep declines are augur buzzard Buteo augur, Dickinson’s kestrel Falco dickinsoni and Beaudouin’s snake-eagle Circaetus beaudouini. The latter is of particular concern, having declined by 80–85% over three generation lengths within a large (and probably representative) portion of its global breeding range52. The plight of these African endemics illustrates the pressing need for research into raptors with restricted breeding ranges.
We show that large African raptors have suffered steeper annual declines than smaller species, mirroring the pattern of extinction risk observed among terrestrial mammalian predators33. The risks to large-bodied species are compounded both by their biological traits (for example, low population density, delayed maturity and low annual fecundity33,53) and environmental factors (home ranges requiring extensive tracts of scarce, suitable habitat, thereby increasing the species’ exposure to human impacts). Furthermore, the loss of large-bodied species has a disproportionate effect on the resilience and functioning of ecosystems, as well as on human-centric values, such as revenue from tourism54,55.
Declines were more pronounced in West Africa
Decline rates reported from West Africa40,56,57 were significantly more pronounced than those recorded elsewhere, consistent with the severity of threats documented in the region31,32,40,56,57,58, many being substantially worse there than elsewhere in sub-Saharan Africa19,59. Protected areas in West and Central Africa are particularly underfunded and mismanaged19, and high regional levels of poverty and corruption have been linked to adverse conservation outcomes for charismatic mammal species59,60. Furthermore, the rate of agricultural expansion in West Africa during the 1970s–2000s was more than three times that of Africa as a whole (Supplementary Information: Anthropogenic pressures). Hence, raptor declines seem likely to have continued in the region since road transect surveys were last conducted in the early 2000s, highlighting the need for repeat surveys. In contrast, SABAP2 reporting rates suggest that proportionately fewer species had declined in South Africa than elsewhere, albeit over a shorter, more recent timeframe (2008–2021).
Migrant species appear to have suffered steeper declines than residents, although this effect was statistically non-significant. Similarly, there was no significant relationship between the direction of change evident among Palaearctic migrants in Africa and in Europe61, perhaps reflecting disparities between the populations surveyed, or shifts in the over-wintering distributions of some Palaearctic migrant species62.
Decline rates were often high within protected areas
Raptors of all sizes lead an increasingly perilous existence in African savannas, where food supplies and breeding sites have been drastically reduced and persecution by humans is now widespread40,42,56,57. While annual declines on unprotected land were thus often substantially higher than within the PAs we assessed, there is now widespread acknowledgement that many African PAs are also losing their ecological integrity18,63,64, thereby depriving threatened species of effective refugia. Indeed, the scale of this deterioration has been assessed in a recent study19, which showed that over 82% of land encompassed within 516 African conservation areas was considered to be failing or deteriorating. Moreover, vulture and eagle species can range widely across protected area boundaries, exposing them to retaliatory and sentinel poisoning by pastoralists and poachers, respectively65, and to persecution by livestock farmers. Consequently, levels of attrition were high even within the PA types we assessed, where 40% of species had declined at rates exceeding the IUCN Vulnerable threshold. Clearly, the size, connectivity and/or management of these PAs has failed to safeguard such highly mobile species, reflecting concerns that many African PAs are too small to protect large raptors adequately66.
Study limitations
While our sample accounted for 40% of Africa’s 106 diurnal raptor species52, their trajectories may not be representative of trends among the remaining species, many of which are forest dependent. Globally, tropical forest raptors are at greater risk of extinction than those associated with savannas8, perhaps especially so in Africa, where net forest loss during 2010–2020 exceeded that of all other continents67. Geographically, North Africa represents a further, notable gap in our coverage. Here, many of the same threats prevail as elsewhere in Africa, and the limited evidence available27,28,29 suggests that raptor population trends in the region may be similar to those south of the Sahara.
Differing trends within PAs and UPAs could result from factors other than site protection, including the possibility that land encompassed within PAs was initially more favourable for raptors than land left unprotected, as indicated by disparities between PA and UPA encounter rates during early survey periods (Supplementary Table 3). To investigate this possibility we re-examined survey data from northern Botswana, demonstrating that PA and UPA encounter rates within the same 1° × 1° grid cells were higher than those from grid cells where PAs were absent, suggesting that high PA encounter rates were due in part to more favourable initial conditions (Supplementary Information: Comparing protected and unprotected areas). However, separating the effects of site protection from other factors would require a more rigorous counterfactual study design68 involving a before–after control–intervention (BACI) approach69, or the careful matching of ecologically similar transects from PAs and UPAs70. The application of a counterfactual approach thus remains the ‘gold standard’ for future analyses of PA effects, and we recommend caution when interpreting PA–UPA disparities.
Shrub encroachment within savanna habitats since the 1980s could have adversely affected raptor detectability, potentially contributing to the disparities observed between early and recent encounter rates. Since vegetation structure in the vicinity of survey transects was not assessed, we were unable to test whether changes in woody cover had occurred along the routes surveyed. Although widespread changes in shrub encroachment have been reported14,71, their effects are likely to have been small in comparison with many of the declines reported here. Moreover, although shrub encroachment would seem less likely to impede the detection of large soaring raptors, these species had shown some of the steepest declines (Supplementary Information: Detectability).
Mitigating raptor declines
While ongoing efforts to protect Africa’s charismatic megafauna, including elephants Loxodonta spp.72 and lions Panthera leo19,57, help safeguard critical raptor habitats, raptors have distinct management requirements differing from those of large mammals. These include the protection of nesting trees and cliffs, the global adoption of bio-pesticides for locust control73, more effective management of Quelea control operations, and an improved understanding of the corridors and habitats required by migrant raptors. Mitigation is urgently required to end the extensive mortality caused by powerlines and windfarms26,27,28,29, particularly along migratory flyways. Innovation is needed to reduce mortalities caused by lethal pole and turbine designs, and better enforcement of regulations is required to prevent energy infrastructure from being built within protected and sensitive areas74.
The future of Africa’s raptors also rests on (1) effective legislation for species protection, (2) enhanced management of PAs, particularly in relation to tree loss, disturbance at nest sites, poaching and poisoning, (3) tighter coordination between government and conservation stakeholders13 and (4) both improved law enforcement and innovative economic incentives to counter persecution24, sentinel poisoning65 and the harvesting of raptors for food and belief-based use30,31,32. Better coordination is also required between range states encompassing migratory routes75, facilitated by frameworks such as the Convention on Migratory Species (CMS) Memorandum of Understanding (MOU) on the conservation of birds of prey in Africa and Eurasia.
To address the need for long-term raptor monitoring and expanded research and conservation programmes, we have developed the African Raptor Leadership Grant, which supports educational and mentoring opportunities, boosting local conservation initiatives and knowledge of raptors across the continent. Furthermore, we recommend increased stakeholder engagement in raptor conservation to develop regional raptor Red Lists, monitoring schemes and species action plans, with guidance from the CMS Raptor MOU Technical Advisory Group and relevant IUCN Species Specialist Groups.
The evidence we present here of a significant shift in the reliance of African raptor species on protected areas substantiates recent calls to expand the global protected area network76,77 and demonstrates the importance of proposals agreed at the Convention on Biological Diversity COP15 in 2022: to effectively conserve and manage at least 30% of the world’s surface by 203078. Furthermore, our results underscore the need to substantially improve PA management throughout Africa, to meet the ‘green list standard’ set by the IUCN World Commission on Protected Areas79. In this regard, a recent African-driven initiative—APACT—may prove pivotal in leveraging the finances needed to effectively manage new and existing conserved areas63.
While raptors also extensively utilize unprotected areas, particularly during migration80 and seasonal stays81, human population projections for sub-Saharan Africa10 point to further, widespread conversion and degradation of natural habitats, particularly on unprotected land. Well-established links between land conversion and biodiversity loss1,2,3,4,9,11, together with the patterns of decline documented here, give cause to doubt whether large raptors will persist over much of Africa’s unprotected land in the latter half of this century. Broad-scale interventions and collaborations are thus urgently required to address the multitude of threats facing raptors in unprotected areas, thereby also helping to protect other wildlife species. Furthermore, there is a pressing need to substantially improve the connectivity, management and coverage of PAs in Africa, in line with global aspirations77,78,79—a transition considered fundamental to safeguarding biodiversity, ecosystem functioning and climate resilience76.
Methods
Road transect studies
We collated published results from road transect studies conducted in Burkina Faso, Niger and Mali (West Africa)40, Kenya (East Africa)42,82 and northern Botswana (southern Africa)20,43, together with published and unpublished survey results from northern Cameroon (Central Africa)41 (R.B. and B.M.C, unpublished data). These studies covered a combined survey distance of 94,151 km (Supplementary Table 1), yielding 53,209 sightings of the 42 study species. In each study, routes were surveyed during an ‘early’ and ‘recent’ period, separated by an interval of ca. 20–40 yr. For each raptor species in each study and survey period, we calculated an average encounter rate (individuals seen per 100 km) separately for routes lying within PAs and UPAs (Extended Data Fig. 1). Protected areas were defined by the authors of the original studies, who excluded site categories affording little or no meaningful protection for wildlife, or where the degree of protection provided was uncertain (Supplementary Information: Survey routes and protected areas). In the absence of historical digital maps, contemporary PA boundaries17 were used when estimating land areas during early and recent survey periods. Where insufficient detail had been provided, PA types were confirmed subsequently by the lead author of the study in question (Supplementary Table 4). To minimize chance effects, we restricted our analyses to species for which at least 20 individuals had been recorded in a given study area during the early survey period, with at least five sightings each in PAs and UPAs. Potential effects of excluding cases with smaller sample sizes are considered in Supplementary Information: Case selection.
Estimating change in encounter rates
We used the following protocol to estimate each species’ annual rate of change within a given study area. First, we averaged its encounter rates within PAs and UPAs separately during the early (E) and the recent period (R). We weighted each average by the extent of land within PAs and UPAs within the species’ range in the study area in question, extracted from the African Raptor Databank83 (Extended Data Fig. 2). Since not all of the selected PAs had been surveyed within a given study area, we estimated each species’ overall encounter rate under two scenarios, in which the average encounter rate within unsurveyed PAs was assumed to have either been (1) the same as in surveyed PAs or (2) the same as in UPAs. These scenarios respectively yielded a high and low estimate of the species’ average encounter rate in each study area and survey period, and hence produced four estimates of change (C) between the two periods. These corresponded to E1→R1, E1→R2, E2→R1 and E2→R2. We converted these to annual rates of change using the formula AC = −(1 − (1 + C)ˆ(1/t)), where ‘AC’ is the annual rate of change, ‘C’ is the overall change between the two periods (replaced by each of the four change estimates in turn), and ‘t’ is the time (in years) separating the midpoints of the two survey periods. This provided four estimates of the annual rate of change of each species in each study.
Fifteen species had been surveyed adequately in just a single study area. For these, we calculated a median annual rate of change from the four estimates. For each of the remaining 27 species, surveyed in multiple studies, we calculated a median annual rate of change by combining one of the n change estimates in turn from each of the relevant studies (Extended Data Fig. 2). Importantly, we weighted each change estimate in accordance with the species’ range size in the study area in question so that extreme changes within a relatively small area (for example, northern Cameroon) did not disproportionately influence the median value. Thus, for species surveyed in two, three or four studies, we calculated a weighted median annual rate of change (AR) from 16, 64 or 256 permutations, respectively. We projected this value (plus quartiles) over three generation lengths (GLs) (ref. 51, R. Martin, personal communication, 2021; Supplementary Table 2) using the formula −(1 − (1 + AR)ˆ(3 × GL)).
In the approach described above, we extrapolated mean encounter rates from surveyed PAs and UPAs to unsurveyed PAs, on the assumption that encounter rates within the latter were likely to be similar to those recorded on surveyed land. To test the effects of these extrapolations, we also estimated rates of change when unsurveyed PAs were excluded from the analyses. Change estimates derived from these two approaches typically differed by just 1–2 percentage points over three generation lengths (median = 1.0; range = 0.1–7.4; n = 42), supporting our decision to use extrapolated values for unsurveyed PAs (Supplementary Table 5 and Fig. 1). Notably, the exclusion of unsurveyed PAs typically yielded decline rates that were slightly more pronounced than those presented in Table 1 and Fig. 2, suggesting that our decline estimates are slightly conservative.
When combining PA and UPA data from multiple studies, we thus weighted annual change rates by the land area surveyed in each study to produce a composite estimate of each species’ rate of change (Extended Data Fig. 2). It was not possible to apply a similar weighting when comparing PA and UPA rates of change due to differences in the relative area of protected and unprotected land present in each study area. For example, most of the protected and unprotected land surveyed occurred in northern Botswana and West Africa, respectively. Had we applied a weighting based on land area, change rates within PAs would have more strongly reflected conditions in northern Botswana, while those in UPAs would have reflected conditions in West Africa. Since declines were significantly more severe in West Africa, this approach would have exaggerated the apparent benefits of site protection. To avoid this potential bias, we compared PA and UPA change rates using unweighted values.
As a measure of each species’ dependency on protected areas, we compared its encounter rates within PAs and UPAs by subtracting the UPA value from the PA value and dividing by the higher value. Thus, if a species’ mean encounter rate within PAs was higher than in UPAs, we calculated its PA dependency index as: (PA rate − UPA rate)/PA rate. Index values potentially ranged from −1.0 (recorded only in UPAs) to +1.0 (recorded only in PAs).
Median body mass values were extracted from ref. 84. In recent African raptor studies, species have been classified as ‘large’ on the basis of a body mass threshold typically set at 1,000–1,400 g21,23,42,43. Following ref. 42, we adopted 1,300 g as the threshold separating these two size groups, partly reflecting their prey requirements, extracted from ref. 85. Among the 42 species surveyed, those weighing ≤1,300 g prey mainly on small mammals, birds, lizards or invertebrates, while the heavier species prey mainly on larger reptiles (particularly snakes), medium-sized birds or mammals, or else scavenge on carcasses (Supplementary Table 2).
We used general linear models (GLMs) and non-parametric tests in R (v.4.1.3)86 to examine changes in species encounter rates in relation to survey period, study area, body mass, protected area status and PA dependency. GLMs were run using the ‘lme4’ package. Where the same species or studies were sampled multiple times, the variables ‘Species’ and/or ‘Study’ were included as random terms. Otherwise, measurements were taken from distinct samples. To avoid over-parameterization, we limited the combined number of explanatory and random variables to two (where n ≥ 42) or three (n ≥ 60). Where appropriate, we compared model variants in which the explanatory variables were either entered separately or as an interaction term. We selected a top model by applying the Akaike information criterion, corrected for small sample sizes (AICc), using ‘AICctab’ in the package ‘bblme’. We used the ‘Anova’ function to calculate Chi-squared and (two-tailed) P values for each explanatory term, and applied the functions ‘testUniformity’, ‘testDispersion’, ‘testOutliers’ and ‘testQuantiles’ in the package ‘DHARMa’ to check that the data complied with model assumptions. Where diagnostics indicated a poor model fit, we instead used a paired Wilcoxon signed-rank test or a Kruskal–Wallis test, as appropriate. Analyses are referred to in the results section as models 1 to 17 in Extended Data Table 1, where each model is summarized.
Determining direction of change from SABAP2 reporting rates
To examine trends among raptors in South Africa, we measured variation in reporting rates during SABAP2 (2007–2021)46 using survey data downloaded from ref. 87, each entry recording the outcome of one visit to one 5’ × 5’ grid cell (pentad). However, interpreting change in SABAP2 reporting rates (the proportion of pentad survey visits yielding at least one sighting of the target species) is problematic, as rates vary in a nonlinear manner in relation to abundance88. We therefore limited our analysis to determining the direction of change. Since relatively few data were collected during 2007, we restricted the dataset to the years 2008–2021. We established that reporting rates tended to increase in relation to visit duration, and decided to limit the dataset to visits of 2–5 h (Supplementary Fig. 2). To ensure adequate survey coverage, we selected pentads that had been surveyed at least 20 times, with a minimum of five visits each in 2008–2014 and 2015–2021. We further limited the dataset to pentads in which the target species was recorded at least twice during the 14-yr period, as confirmation of occupancy. Of the 42 species examined, 30 met these selection criteria within at least 30 pentads in South Africa (Extended Data Table 2).
We used the ‘glmer’ function in R to determine, for each species in turn, whether SABAP2 reporting rates varied significantly in relation to year. We specified the target species’ detection during pentad visits as the dependent variable (binary: positive, negative) and ‘Year’ (numeric: 08–21) as a fixed effect, fitting each model with a binomial error distribution. Since reporting rates tend to vary seasonally, we also entered ‘Seasonal interval’ as a fixed effect, dividing the calendar year into 6, 4, 3, 2 or 1-month intervals in separate model variants. Since each pentad was sampled multiple times, ‘Pentad ID’ was entered as a random effect. We selected a top model on the basis of the minimum AICc value. Where the AICc values for model variants differed by no more than 2 points we selected the variant in which ‘Seasonal interval’ was more finely resolved, for example, into 12 calendar months rather than six 2-month intervals. The direction of change in reporting rates was determined from the slope coefficient, and Chi-squared and P values were calculated using the ‘Anova’ function (Extended Data Table 2).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Survey data used in statistical analyses are available in Figshare, with the identifier https://doi.org/10.6084/m9.figshare.23727030. Additional background data used in the study are available in the Supplementary Information. Source data are provided with this paper.
Code availability
Statistical analyses were conducted using open-source packages and functions in R. Copies of the code used to reformat data and perform analyses are available in Figshare, with the identifier https://doi.org/10.6084/m9.figshare.23727030.
References
Foley, J. et al. Global consequences of land use. Science 309, 570–574 (2005).
Green, R. E., Cornell, S. J., Scharlemann, J. P. W. & Balmford, A. Farming and the fate of wild nature. Science 307, 550–555 (2005).
Jetz, W., Wilcove, D. S. & Dobson, A. P. Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 5, e157 (2007).
Balmford, A., Green, R. & Phalan, B. What conservationists need to know about farming. Proc. R. Soc. B 279, 2714–2724 (2012).
Newton, I. Population Ecology of Raptors (T & AD Poyser,1979).
Simmons, R. E. Harriers of the World: Their Behaviour and Ecology (OUP, 2000).
Brown, L. H. The conservation of African birds: threats, problems and action needed. In Proc. 4th Pan-African Ornithological Congress (ed. Johnson, D. N.) 345–354 (Southern African Ornithological Society, 1980).
McClure, C. J. et al. State of the world’s raptors: distributions, threats, and conservation recommendations. Biol. Conserv. 227, 390–402 (2018).
O’Bryan, C. J. et al. Human impacts on the world’s raptors. Front. Ecol. Evol. 10, 624896 (2022).
World Population Prospects 2022 (United Nations, 2023).
Carrete, M., Tella, J. L., Blanco, G. & Bertellotti, M. Effects of habitat degradation on the abundance, richness and diversity of raptors across Neotropical biomes. Biol. Conserv. 142, 2002–2011 (2009).
Butchart, S. H. et al. Shortfalls and solutions for meeting national and global conservation area targets. Conserv. Lett. 8, 329–337 (2015).
Amar, A., Buij, R., Suri, J., Sumasgutner, P. & Virani, M. Z. in Birds of Prey (eds Sarasola, J. et al.) 419–455 (Springer, 2018).
Brink, A. B. & Eva, H. D. Monitoring 25 years of land cover change dynamics in Africa: a sample-based remote sensing approach. Appl. Geogr. 29, 501–512 (2009).
Nkonya, E., Johnson, T., Kwon, H. Y. & Kato, E. in Economics of Land Degradation and Improvement – A Global Assessment for Sustainable Development (eds Nkonya, E. et al.) 215–260 (Springer, 2016).
Bruinsma, J. The Resource Outlook to 2050: By How Much Do Land, Water and Crop Yields Need to Increase by 2050? Expert Meeting on How to Feed the World in 2050 (FAO, 2009).
Protected Area Profile for Africa from the World Database on Protected Areas (UNEP-WCMC, March 2023); www.protectedplanet.net
Tranquilli, S. et al. Protected areas in tropical Africa: assessing threats and conservation activities. PLoS ONE 9, e114154 (2014).
Robson, M. T. et al. Over 80% of Africa’s savanna conservation land is failing or deteriorating according to lions as an indicator species. Conserv. Lett. 15, e12844 (2022).
Herremans, M. & Herremans-Tonnoeyr, D. Land use and the conservation status of raptors in Botswana. Biol. Conserv. 94, 31–41 (2000).
Buij, R., Croes, B. M., Gort, G. & Komdeur, J. The role of breeding range, diet, mobility and body size in associations of raptor communities and land-use in a West African savanna. Biol. Conserv. 166, 231–246 (2013).
Amar, A. & Cloete, D. Quantifying the decline of the martial eagle Polemaetus bellicosus in South Africa. Bird. Conserv. Int. 28, 363–374 (2018).
Shaw, P. et al. Implications of farmland expansion for species abundance, richness and mean body mass in African raptor communities. Biol. Conserv. 235, 164–177 (2019).
Madden, K. K., Rozhon, G. C. & Dwyer, J. F. Conservation letter: raptor persecution. J. Raptor Res. 53, 230–233 (2019).
Green, R. E., Pain, D. J. & Krone, O. The impact of lead poisoning from ammunition sources on raptor populations in Europe. Sci. Total Environ. 823, 154017 (2022).
Eccleston, D. T. & Harness, R. E. in Birds of Prey (eds Sarasola, J. H. et al.) 273–302 (Springer, 2018).
Irizi, A., Aourir, M., El Agbani, M. A. & Qninba, A. Correlates of persistent electrocution-related mortality of raptors in Guelmim-Oued Noun province, Morocco. Ostrich 92, 85–93 (2021).
Oppel, S. et al. Major threats to a migratory raptor vary geographically along the eastern Mediterranean flyway. Biol. Conserv. 262, 109277 (2021).
Garrido, J. R. et al. The Conservation Status and Distribution of the Breeding Birds of Prey of North Africa (IUCN, 2021).
Whytock, R. C., Buij, R., Virani, M. Z. & Morgan, B. J. Do large birds experience previously undetected levels of hunting pressure in the forests of Central and West Africa? Oryx 50, 76–83 (2014).
Buij, R., Nikolaus, G., Whytock, R., Ingram, D. J. & Ogada, D. Trade of threatened vultures and other raptors for fetish and bushmeat in West and Central Africa. Oryx 50, 606–616 (2016).
Henriques, M. et al. Deliberate poisoning of Africa’s vultures. Science 370, 6514 (2020).
Ripple, W. J. et al. Status and ecological effects of the world’s largest carnivores. Science 343, 1241484 (2014).
Estes, J. A. et al. Trophic downgrading of planet Earth. Science 6040, 301–306 (2011).
Dirzo, R. et al. Defaunation in the anthropocene. Science 345, 401–406 (2014).
Buechley, E. T. & Şekercioğlu, C. H. The avian scavenger crisis: looming extinctions, trophic cascades, and loss of critical ecosystem functions. Biol. Conserv. 198, 220–228 (2016).
O’Bryan, C. J. et al. The contribution of predators and scavengers to human well-being. Nat. Ecol. Evol. 2, 229–236 (2018).
Markandya, A. et al. Counting the cost of vulture decline – an appraisal of the human health and other benefits of vultures in India. Ecol. Econ. 67, 194–204 (2008).
Ogada, D. L., Torchin, M. E., Kinnaird, M. F. & Ezenwa, V. O. Effects of vulture declines on facultative scavengers and potential implications for mammalian disease transmission. Conserv. Biol. 26, 453–460 (2012).
Thiollay, J. M. The decline of raptors in West Africa: long-term assessment and the role of protected areas. Ibis 148, 240–254 (2006).
Thiollay, J. M. Long-term changes of raptor populations in Northern Cameroon. J. Raptor Res. 35, 173–186 (2001).
Ogada, D. et al. Evidence of widespread declines in Kenya’s raptor populations over a 40-year period. Biol. Conserv. 266, 109361 (2022).
Garbett, R., Herremans, M., Maude, G., Reading, R. P. & Amar, A. Raptor population trends in northern Botswana: a re-survey of road transects after 20 years. Biol. Conserv. 224, 87–99 (2018).
Guidelines for Using the IUCN Red List Categories and Criteria Version 15.14 (IUCN, 2022).
Data Zone (BirdLife International, 2022); http://datazone.birdlife.org/species/search
Brooks, M. et al. The African Bird Atlas Project: a description of the project and BirdMap data-collection protocol. Ostrich https://doi.org/10.2989/00306525.2022.2125097 (2022).
Ogada, D. et al. Another continental vulture crisis: Africa’s vultures collapsing toward extinction. Conserv. Lett. 9, 89–97 (2016).
Hofmeyr, S. D., Symes, C. T. & Underhill, L. G. Secretarybird Sagittarius serpentarius population trends and ecology: insights from South African citizen science data. PLoS ONE 9, e96772 (2014).
Leepile, L. B. et al. Changes in nesting numbers and breeding success of African white-backed vulture Gyps africanus in north-central Botswana. Bird. Conserv. Int. 30, 456–473 (2020).
Eichenwald, A. J., Amar, A., Tyrrell, P., Buechley, E. R. & Virani, M. Z. Declines in an augur buzzard Buteo augur population in a region of increasing human development. Front. Ecol. Evol. 9, 109 (2021).
Bird, J. P. et al. Generation lengths of the world’s birds and their implications for extinction risk. Conserv. Biol. 34, 1252–1261 (2020).
Clark, B. & Davies. R. African Raptors (Bloomsbury Publishing, 2018).
Cardillo, M. et al. Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241 (2005).
Di Marco, M. et al. A retrospective evaluation of the global decline of carnivores and ungulates. Conserv. Biol. 28, 1109–1118 (2014).
Ripple, W. J. et al. Collapse of the world’s largest herbivores. Sci. Adv. 1, e1400103 (2015).
Thiollay, J. M. Severe decline of large birds in the Northern Sahel of West Africa: a long-term assessment. Bird. Conserv. Int. 16, 353–365 (2006).
Thiollay, J. M. Large bird declines with increasing human pressure in savanna woodlands (Burkina Faso). Biodivers. Conserv. 15, 2085–2108 (2006).
Henriques, M. et al. Status of birds of prey in Guinea-Bissau: first assessment based on road surveys. Ostrich 88, 101–111 (2017).
Lindsey, P. A. The performance of African protected areas for lions and their prey. Biol. Conserv. 209, 137–149 (2017).
Hauenstein, S., Kshatriya, M., Blanc, J., Dormann, C. F. & Beale, C. M. African elephant poaching rates correlate with local poverty, national corruption and global ivory price. Nat. Comm. 10, 2242 (2019).
European Red List of Birds (BirdLife International, 2021); https://www.birdlife.org/wp-content/uploads/2021/10/BirdLife-European-Red-List-of-Birds-2021.pdf
Howes, C., Symes, C. T. & Byholm, P. Evidence of large‐scale range shift in the distribution of a Palaearctic migrant in Africa. Divers. Distrib. 25, 1142–1155 (2019).
Bakarr, M. I. Reimagining protected and conserved areas in Africa: perspectives from the first Africa Protected Areas Congress. Conserv. Lett. 16, e12944 (2023).
Obura, D. O. et al. Integrate biodiversity targets from local to global levels. Science 373, 746–748 (2021).
Ogada, D., Botha, A. & Shaw, P. Ivory poachers and poison: drivers of Africa’s declining vulture populations. Oryx 50, 593–596 (2016).
Murn, C. et al. Using Africa’s protected area network to estimate the global population of a threatened and declining species: a case study of the critically endangered white-headed vulture Trigonoceps occipitalis. Ecol. Evol. 6, 1092–1103 (2016).
The State of the World’s Forests 2020. Forests, Biodiversity and People (FAO and UNEP, 2020); https://doi.org/10.4060/ca8642en
Wauchope, H. S. et al. Protected areas have a mixed impact on waterbirds, but management helps. Nature 605, 103–107 (2022).
Wauchope, H. S. et al. Evaluating impact using time-series data. Trends Ecol. Evol. 36, 196–205 (2021).
Terraube, J., Van doninck, J., Helle, P. & Cabeza, M. Assessing the effectiveness of a national protected area network for carnivore conservation. Nat. Commun. 11, 2957 (2020).
Venter, Z. S., Cramer, M. D. & Hawkins, H.-J. Drivers of woody plant encroachment over Africa. Nat. Comm. 9, 2272 (2018).
Wittemyer, G. et al. Illegal killing for ivory drives global decline in African elephants. Proc. Natl Acad. Sci. USA 111, 13117–13121 (2014).
Mullié, W. C., Prakash, A., Müller, A. & Lazutkaite, E. Insecticide use against desert locust in the Horn of Africa 2019–2021 reveals a pressing need for change. Agronomy 13, 819 (2023).
Cervantes, F. et al. A utilization distribution for the global population of Cape vultures (Gyps coprotheres) to guide wind energy development. Ecol. Appl. 33, e2809 (2023).
Botha, A. J. et al. Multi-species Action Plan to Conserve African-Eurasian Vultures Convention on Migratory Species, Raptors MOU Technical Publication No. 5 (Coordinating Unit of the Raptors MOU, 2017).
IPCC: Summary for Policymakers. In Climate Change 2022: Impacts, Adaptation and Vulnerability (eds Pörtner, H.-O. et al.) (Cambridge Univ. Press, 2022).
Protecting the Planet 30×30 IUCN Policy Brief March 2022 (IUCN, 2022).
COP15: Nations Adopt Four Goals, 23 Targets for 2030 in Landmark UN Biodiversity Agreement (Convention on Biological Diversity, 2022).
IUCN Green List of Protected and Conserved Areas: Standard Version 1.1 (IUCN and WCPA, 2017).
Oppel, S. et al. High juvenile mortality during migration in a declining population of a long‐distance migratory raptor. Ibis 157, 545–557 (2015).
Kassara, C. et al. Current and future suitability of wintering grounds for a long-distance migratory raptor. Sci. Rep. 7, 8798 (2017).
Virani, M. Z., Kendall, C., Njoroge, P. & Thomsett, S. Major declines in the abundance of vultures and other scavenging raptors in and around the Masai Mara ecosystem, Kenya. Biol. Conserv. 144, 746–752 (2011).
African Raptor Databank February 2013 (HabitatInfo, accessed 30 September 2019); http://www.habitatinfo.com/wp-content/uploads/2013/02/ARDB-Factsheet.pdf
del Hoyo, J., Elliott, A., Sargatal, J., Christie, D. A. & de Juana, E. Handbook of the Birds of the World (Lynx Edicions, 2019).
Brown, L. African Birds of Prey (Collins, 1970).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2022).
Southern African Bird Atlas Project (SABAP2, 2022).
Lee, A. T. K., Fleming, C. & Wright, D. R. Modelling bird atlas reporting rate as a function of density in the southern Karoo, South Africa. Ostrich 89, 363–372 (2018).
Acknowledgements
This study would not have been possible without the survey work undertaken by the late J. M. Thiollay and C. Smeenk in West and East Africa, respectively. Funding sources for the four road transect studies have been acknowledged in the relevant publications20,40,41,42,43,82. Additional data were incorporated into the present study from surveys conducted in Cameroon by R.B. and B.M.C.; these were financially and logistically supported by the Institute of Environmental Sciences (CML) of the University of Leiden, the Netherlands, through its collaborative programme with the University of Dschang, Cameroon, at the Centre for Environment and Development Studies in Cameroon (CEDC). In addition, D.O. acknowledges logistical support from the National Geographic Society and San Diego Zoo Wildlife Alliance. P.S. gratefully acknowledges support received from the University of St Andrews, at which he is an Honorary Research Fellow. We also thank R. Davies and his team at Habitat Info for providing up-to-date range maps for African raptors; R. Patchett for advice on modelling road transect data; R. Camp for advice on weighting methods; the many citizen scientists who have contributed to SABAP2; and M. Brooks for guidance on accessing SABAP2 data.
Author information
Authors and Affiliations
Contributions
P.S. and D.O. conceived the study and collated published and unpublished road transect data. D.O., R.B., R.G., M.H., M.Z.V., C.J.K., B.M.C., M.O., S.K., P.W., G.M. and S.T. collected data. L.D. performed the analysis of species and PA distributions. P.S. analysed the road transect data. A.A. and P.S. formulated the analysis of SABAP2 data, which P.S. performed. P.S. and D.O. wrote the paper, with contributions from C.R., A.A., R.B., M.H., A.B., M.Z.V., U.G.-O., C.M. and C.J.K., who helped finalize the text.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Chevonne Reynolds and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Routes surveyed during four road transect studies.
These were conducted during 1969–1977 and 2000–2020 in northern Cameroon, Kenya, Burkina Faso, Niger and Mali, and during 1991–1995 and 2015–2016 in northern Botswana. Panels adapted with permission from: Burkina Faso, Niger and Mali, ref. 40, Wiley; northern Cameroon, ref. 41, Allen Press; Kenya, ref. 42, Elsevier; northern Botswana, ref. 43, Elsevier.
Extended Data Fig. 2 Method used to produce a composite estimate of the rate of change in abundance.
The survey data used here are for bateleur Terathopius ecaudatus, and were drawn from all four road transect studies. Average encounter rates within PAs and UPAs are shown for early (E) and recent (R) survey periods. For each period, we combined these to produce a weighted average for the study area in question, based on two scenarios, in which the average encounter rate within unsurveyed PAs was assumed either to be (1.) the same as in surveyed PAs, or (2.) the same as in UPAs. The land area to which the PA encounter rate was assumed to apply thus differed between these two scenarios, as indicated by the relative sizes of the green (PA) and red (UPA) boxes shown, exaggerated here for illustrative effect. These encounter rate values yielded four estimates of change for each study area between survey periods, corresponding to E1– > R1, E1– > R2, E2– > R1 and E2– > R2, as illustrated. We converted these estimates to annual rates of change for each study area, and multiplied them by the species’ range size within each area. We used the weighted values to calculate an average annual rate of change for each of the 256 permutations, derived from the four change estimates and four study areas. Finally, we calculated the median plus quartiles 1 and 3 from these permutations, and projected these over three generation lengths.
Supplementary information
Supplementary Information
Extended Methods, Supplementary Tables 1–9, Figs. 1–3 and References.
Source data
Source Data Fig. 1
Source data for regional bar charts, showing percentage decline in bateleur encounter rates within protected and unprotected areas, projected over three generation lengths.
Source Data Fig. 2
Source data for horizontal bar chart, showing percentage change in encounter rates for 42 species, projected over three generation lengths.
Source Data Fig. 3
Source data for bubble chart, showing change rates over three generation lengths, in relation to body mass.
Source Data Fig. 4
Source data for Fig 4 showing: decline rates within regions, in relation to PA status; change rates in relation to body mass and PA status; modelled change rates within PAs and UPAs, in relation to body mass.
Source Data Fig. 5
Source data for Fig. 5 showing change in PA dependency values in relation to body size class, and change in PA dependency in relation to change in abundance.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaw, P., Ogada, D., Dunn, L. et al. African savanna raptors show evidence of widespread population collapse and a growing dependence on protected areas. Nat Ecol Evol 8, 45–56 (2024). https://doi.org/10.1038/s41559-023-02236-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-023-02236-0