Abstract
Secondary forests constitute an increasingly important component of tropical forests worldwide. Although cycling of essential nutrients affects recovery trajectories of secondary forests, the effect of nutrient limitation on forest regrowth is poorly constrained. Here we use three lines of evidence from secondary forest succession sequences in central Africa to identify potential nutrient limitation in regrowing forests. First, we show that atmospheric phosphorus supply exceeds demand along forest succession, whereas forests rely on soil stocks to meet their base cation demands. Second, soil nutrient metrics indicate that available phosphorus increases along the succession, whereas available cations decrease. Finally, fine root, foliar and litter stoichiometry show that tissue calcium concentrations decline relative to those of nitrogen and phosphorus during succession. Taken together, these observations suggest that calcium becomes an increasingly scarce resource in central African forests during secondary succession. Furthermore, ecosystem calcium storage shifts from soil to woody biomass over succession, making it a vulnerable nutrient in the wake of land-use change scenarios that involve woody biomass export. Our results thus call for a broadened focus on elements other than nitrogen and phosphorus regarding tropical forest biogeochemical cycles and identify calcium as a scarce and potentially limiting nutrient in an increasingly disturbed and dynamic tropical forest landscape.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data to generate figures and tables are available from https://doi.org/10.6084/m9.figshare.19697353.
References
Losos, E. & Leigh, E. G. Tropical Forest Diversity and Dynamism: Findings from a Large-Scale Plot Network (Univ. Chicago Press, 2004).
Pan, Y. et al. A large and persistent carbon sink in the world’s forests. Science 333, 988–993 (2011).
Hansen, M. C. C. et al. High-resolution global maps of 21st-century forest cover change. Science 342, 850–854 (2013).
Chazdon, R. L. Beyond deforestation: restoring degraded lands. Science 1458, 1458–1460 (2008).
Global Forest Resources Assessment 2010 (FAO, 2010).
Rozendaal, D. M. A. & Chazdon, R. L. Demographic drivers of tree biomass change during secondary succession in northeastern Costa Rica. Ecol. Appl. 25, 506–516 (2015).
Poorter, L. et al. Biomass resilience of Neotropical secondary forests. Nature 530, 211–214 (2016).
Chazdon, R. L., Broadbent, E. N., Rozendaal, D. M. A., Bongers, F. & Al, E. Carbon sequestration potential of second-growth forest regeneration in the Latin American tropics. Sci. Adv. 2, e1501639 (2016).
Lohbeck, M. et al. Functional diversity changes during tropical forest succession. Perspect. Plant Ecol. Evol. Syst. 14, 89–96 (2012).
Poorter, L. et al. Wet and dry tropical forests show opposite successional pathways in wood density but converge over time. Nat. Ecol. Evol. 3, 928–934 (2019).
Townsend, A. R., Cleveland, C. C., Houlton, B. Z., Alden, C. B. & White, J. W. Multi-element regulation of the tropical forest carbon cycle. Front. Ecol. Environ. 9, 9–17 (2011).
Medvigy, D. et al. Observed variation in soil properties can drive large variation in modelled forest functioning and composition during tropical forest secondary succession. New Phytol. 223, 1820–1833 (2019).
Powers, J. S., Mar, E. & Marín-Spiotta, E. Ecosystem processes and biogeochemical cycles during secondary tropical forest succession. Annu. Rev. Ecol. Evol. Syst. 48, 497–519 (2017).
Davidson, E. A. et al. Recuperation of nitrogen cycling in Amazonian forests following agricultural abandonment. Nature 447, 995–998 (2007).
Davidson, E. A. & Martinelli, L. A. in Amazonia and Global Change (eds Keller, M. et al.) 299–309 (American Geophysical Union, 2013).
Vitousek, P. M. & Howarth, R. W. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13, 87–115 (1991).
Batterman, S. A. et al. Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature 502, 224–227 (2013).
Bauters, M., Mapenzi, N., Kearsley, E., Vanlauwe, B. & Boeckx, P. Facultative nitrogen fixation by legumes in the central Congo basin is downregulated during late successional stages. Biotropica 48, 281–284 (2016).
Van Langenhove, L. et al. Regulation of nitrogen fixation from free-living organisms in soil and leaf litter of two tropical forests of the Guiana shield. Plant Soil 450, 93–110 (2020).
Vitousek, P. M. Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 65, 285–298 (1984).
Kaspari, M. et al. Multiple nutrients limit litterfall and decomposition in a tropical forest. Ecol. Lett. 11, 35–43 (2008).
Cleveland, C. C. et al. Relationships among net primary productivity, nutrients and climate in tropical rain forest: a pan-tropical analysis. Ecol. Lett. 14, 939–947 (2011).
Chadwick, O. A., Derry, L. A., Vitousek, P. M., Huebert, B. J. & Hedin, L. O. Changing sources of nutrients during four million years of ecosystem development. Nature 397, 491–497 (1999).
Hedin, L. O. et al. Nutrient losses over four million years of tropical forest development. Ecology 84, 2231–2255 (2003).
Sanchez, P. A., Villachica, J. H. & Bandy, D. E. Soil fertility dynamics after clearing a tropical rainforest in Peru. Soil Sci. Soc. Am. J. 47, 1171 (1983).
Davidson, E. A. et al. Nitrogen and phosphorus limitation of biomass growth in a tropical secondary forest. Ecol. Appl. 14, 150–163 (2004).
Wardle, D. A., Walker, L. R. & Bardgett, R. D. Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 305, 509–513 (2004).
Wassen, M. J., Venterink, H. O., Lapshina, E. D. & Tanneberger, F. Endangered plants persist under phosphorus limitation. Nature 437, 547–550 (2005).
Waring, B. G., Becknell, J. M. & Powers, J. S. Nitrogen, phosphorus, and cation use efficiency in stands of regenerating tropical dry forest. Oecologia 178, 887–897 (2015).
De longe, M., D’odorico, P. & Lawrence, D. Feedbacks between phosphorus deposition and canopy cover: the emergence of multiple stable states in tropical dry forests. Glob. Change Biol. 14, 154–160 (2008).
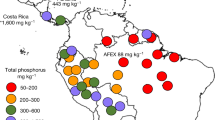
Bauters, M. et al. Fire-derived phosphorus fertilization of African Tropical Forests. Nat. Commun. 12, 5129 (2021).
Vitousek, P. M. & Reiners, W. A. Ecosystem succession and nutrient retention: a hypothesis. Bioscience 25, 376–381 (1975).
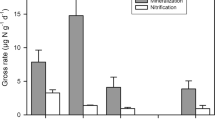
Gallarotti, N. et al. In-depth analysis of N 2O fluxes in tropical forest soils of the Congo Basin combining isotope and functional gene analysis. ISME J. 15, 3357–3374 (2021).
Gorham, E., Vitousek, P. M. & Reiners, W. A. The regulation of chemical budgets over the course of terrestrial ecosystem succession. Annu. Rev. Ecol. Syst. 10, 53–84 (1979).
Markewitz, D., Davidson, E., Moutinho, P. & Nepstad, D. Nutrient loss and redistribution after forest clearing on a highly weathered soil in Amazonia. Ecol. Appl. 14, 177–199 (2004).
Lawrence, D. et al. Ecological feedbacks following deforestation create the potential for a catastrophic ecosystem shift in tropical dry forest. Proc. Natl Acad. Sci. USA 104, 20696–20701 (2007).
Veldkamp, E., Schmidt, M., Powers, J. S. & Corre, M. D. Deforestation and reforestation impacts on soils in the tropics. Nat. Rev. Earth Environ. 1, 590–605 (2020).
Sanchez, P. A. Properties and Management of Soils in the Tropics (John Wiley and Sons, 1976).
Turner, B. L. & Engelbrecht, B. M. J. Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry 103, 297–315 (2011).
Sullivan, B. W. et al. Biogeochemical recuperation of lowland tropical forest during succession. Ecology 100, e02641 (2019).
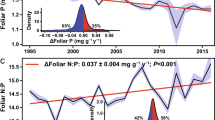
Sardans, J. et al. Empirical support for the biogeochemical niche hypothesis in forest trees. Nat. Ecol. Evol. 13, 184–194 (2021).
White, P. J. & Broadley, M. R. Calcium in plants. Ann. Bot. 92, 487–511 (2003).
Vitousek, P. M., Porder, S., Houlton, B. Z. & Chadwick, O. A. Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 20, 5–15 (2010).
Huggett, B. A., Schaberg, P. G., Hawley, G. J. & Eagar, C. Long-term calcium addition increases growth release, wound closure, and health of sugar maple (Acer saccharum) trees at the Hubbard Brook Experimental Forest. Can. J. For. Res. 37, 1692–1700 (2007).
Marschner, P. Marschner’s Mineral Nutrition of Higher Plants 3rd edn (Elsevier/Academic Press 2002).
Walker, L. R., Wardle, D. A., Bardgett, R. D. & Clarkson, B. D. The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 98, 725–736 (2010).
Bauters, M. et al. Soil nutrient depletion and tree functional composition shift following repeated clearing in secondary forests of the Congo Basin. Ecosystems 24, 1422–1435 (2021).
Turner, B. L., Brenes-arguedas, T. & Condit, R. Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature 555, 367–370 (2018).
Wright, S. J. Plant responses to nutrient addition experiments conducted in tropical forests. Ecol. Monogr. 89, e01382 (2019).
Lugli, L. F. et al. Rapid responses of root traits and productivity to phosphorus and cation additions in a tropical lowland forest in Amazonia. New Phytol. 230, 116–128 (2021).
Vitousek, P. M. M. & Sanford, R. L. Nutrient cycling in moist tropical forest. Annu. Rev. Ecol. Syst. 17, 137–167 (1986).
Kaspari, M. & Powers, J. S. Biogeochemistry and geographical ecology: embracing all twenty-five elements required to build organisms. Am. Nat. 188, S62–S73 (2016).
Nykvist, N. in Soils of Tropical Forest Ecosystems (eds Schulte, A. & Ruhiyat, D.) 87–91 (Springer, 1998).
Bunyavejchewin, S., Sinbumroong, A., Turner, B. L. & Davies, S. J. Natural disturbance and soils drive diversity and dynamics of seasonal dipterocarp forest in Southern Thailand. J. Trop. Ecol. 35, 95–107 (2019).
Quesada, C. A. et al. Variations in chemical and physical properties of Amazon forest soils in relation to their genesis. Biogeosciences 7, 1515–1541 (2010).
Gerland, P. et al. World population stabilization unlikely this century. Science 346, 234–237 (2014).
Makelele, I. A. et al. Afrotropical secondary forests exhibit fast diversity and functional recovery, but slow compositional and carbon recovery after shifting cultivation. J. Veg. Sci. 32, e13071 (2021).
Van Langenhove, L. et al. Atmospheric deposition of elements and its relevance for nutrient budgets of tropical forests. Biogeochemistry 149, 175–193 (2020).
Staelens, J. et al. Calculating dry deposition and canopy exchange with the canopy budget model: review of assumptions and application to two deciduous forests. Water Air Soil Pollut. 191, 149–169 (2008).
Hofhansl, F. et al. Topography strongly affects atmospheric deposition and canopy exchange processes in different types of wet lowland rainforest, southwest Costa Rica. Biogeochemistry 106, 371–396 (2011).
Schrijver, A. De, Nachtergale, L. & Staelens, J. Comparison of throughfall and soil solution chemistry between a high-density Corsican pine stand and a naturally regenerated silver birch stand. Environ Pollut. 131, 93–105 (2004).
Eriksson, E. & Khunakasem, V. Chloride concentration in groundwater, recharge rate and rate of deposition of chloride in the Israel coastal plain. J. Hydrol. 7, 178–197 (1969).
Malhi, Y. et al. An international network to monitor the structure, composition and dynamics of Amazonian forests (RAINFOR). J. Veg. Sci. 13, 439 (2002).
Réjou-Méchain, M., Tanguy, A., Piponiot, C., Chave, J. & Hérault, B. biomass: an R package for estimating above-ground biomass and its uncertainty in tropical forests. Methods Ecol. Evol. 8, 1163–1167 (2017).
Chave, J. et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Change Biol. 20, 3177–3190 (2014).
Malhi, Y. et al. The Global Ecosystems Monitoring network: monitoring ecosystem productivity and carbon cycling across the tropics. Biol. Conserv. 253, 108889 (2021).
D’Angelo, E., Crutchfield, J. & Vandiviere, M. Rapid, sensitive, microscale determination of phosphate in water and soil. J. Environ. Qual. 30, 2206–2209 (2001).
Rowland, A. P. & Haygarth, P. M. Determination of total dissolved phosphorus in soil solutions. J. Environ. Qual. 26, 410–415 (1997).
Vance, E. D., Brookes, P. C. & Jenkinson, D. S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707 (1987).
Brookes, P. C., Powlson, D. S. & Jenkinson, D. S. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 14, 319–329 (1982).
Kaiser, C. et al. Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytol. 187, 843–858 (2010).
Pérez-Harguindeguy, N. et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 61, 167–234 (2013).
Poorter, L. et al. Multidimensional tropical forest recovery. Science 374, 1370–1376 (2021).
Acknowledgements
M.B. is funded by the Fonds Wetenschappelijk Onderzoek (FWO) Flanders, through a postdoctoral fellowship. We thank the people of the Yoko village for the long-lasting collaboration and safeguarding of our equipment in the experimental forest.
Author information
Authors and Affiliations
Contributions
M. Bauters, P.B., K.V. and I.A.J. conceived the study. I.A.M., M. Bauters and C.E. carried out and oversaw the field work and set up the permanent sampling units, with help for maintenance from S.B., T.W.D. and M. Barthel. M.G., D.W. and S.D. analysed the soil samples. P.V. analysed the plant tissue samples. M. Bauters, P.B., K.V.O., I.A.M., J.S., M. Barthel, C.E., T.W.D. and S.B. have provided logistical support for the field work. M. Bauters analysed the data and wrote the paper, with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Helena Vallicrosa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bauters, M., Janssens, I.A., Wasner, D. et al. Increasing calcium scarcity along Afrotropical forest succession. Nat Ecol Evol 6, 1122–1131 (2022). https://doi.org/10.1038/s41559-022-01810-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-022-01810-2
This article is cited by
-
Response of tropical forest productivity to seasonal drought mediated by potassium and phosphorus availability
Nature Geoscience (2024)
-
Nutrient resorption efficiency of twigs is more vulnerable to warming than that of leaves of Cunninghamia lanceolata seedlings
Plant and Soil (2024)
-
Precipitation and plant functional composition mediate desert canopy nutrient responses to water and nitrogen addition
Plant and Soil (2024)
-
Calcium availability affects the intrinsic water-use efficiency of temperate forest trees
Communications Earth & Environment (2023)
-
Nitrogen-bedrock interactions regulate multi-element nutrient limitation and sustainability in forests
Biogeochemistry (2023)