Abstract
The degree to which the distribution of mangrove forests will be impacted by climate change depends on the dispersal and establishment of sea-faring propagules, which drive forest rejuvenation, gene flow and range expansion. Climate change affects sea surface density via changes in temperature and salinity. However, these changes have not been mapped and it remains unclear how these factors may impact mangrove propagule dispersal. Here, we provide evidence for strong warming of coastal mangrove waters and elevated geographic variability in surface ocean density under representative concentration pathway RCP 8.5 by 2100. The largest changes will occur in the Indo West Pacific region, the primary hotspot of mangrove diversity. By comparing propagule densities to predicted sea surface density, we assessed potential effects on mangrove propagule dispersal. In the future, a warmer and fresher ocean is likely to alter dispersal trajectories of mangrove propagules and increase rates of sinking in unsuitable offshore locations, potentially reducing the resilience of mangrove forests.
Similar content being viewed by others
Main
Mangrove forests thrive along tropical and subtropical shorelines and their distribution extends to warm temperate regions1. They are globally recognized for the valuable ecosystem services they provide2 but are expected to be substantially influenced by climate change-related physical processes in the future3,4. Under warming winter temperatures, poleward expansion is predicted for mangroves5,6, with potential implications for ecosystem structure and functioning, as well as human livelihoods and well-being7,8. The global distribution, abundance and species richness of mangroves is governed by a broad range of biotic and environmental factors, including temperature and precipitation9 and diverse geomorphological and hydrological gradients10. Climate and aspects related to coastal geography (for example, floodplain area) determine the availability of suitable habitat for establishment11,12. However, the potential for mangroves to track changing environmental conditions and expand their distributions ultimately depends on dispersal11,13. The importance of dispersal in controlling mangrove distributions has been demonstrated by mangrove distributional responses to historical climate variability14, past mangrove (re)colonization of oceanic islands15 and from the long-term survival of mangrove seedlings planted beyond natural range limits16. As such, quantifying changes in the factors that influence dispersal is important for understanding climate-driven distributional responses of mangroves under future climate conditions.
In mangroves, dispersal is accomplished by buoyant seeds and fruits (hereafter referred to as ‘propagules’). In combination with prevailing currents, the spatial scale of this process, ranging from local retention to transoceanic dispersal over thousands of kilometres13, is determined by propagule buoyancy17, that is, the density difference between that of propagules and the surrounding water. Hence, the course of dispersal trajectories for propagules from these species depends on the interaction between spatiotemporal changes in both propagule density and that of the surrounding water, rendering this process sensitive to climate-driven changes in coastal and open-ocean water properties. The biogeographic implications of such density differences were recognized more than a century ago by Henry Brougham Guppy, who discussed18 ‘the far-reaching influence on plant-distribution and on plant-development that the relation between the specific weight of seeds and fruits and the density of sea-water must possess’.
Since the time of Guppy’s early observations, climate change from human activities has driven pronounced changes in ocean temperature and salinity, with further changes predicted throughout the twenty-first century19. Ocean density is a nonlinear function of temperature, salinity and pressure20; therefore, these changes may influence dispersal patterns of mangrove propagules by altering their buoyancy and floating orientation. As Guppy noted18, ‘[for] plants whose seeds or fruits are not much lighter than seawater […] the effect of increased density of the water is to extend the flotation period’ or ‘to increase the number that floated for a given period’. Guppy also reported that the seedlings of the widespread mangrove genera Rhizophora and Bruguiera present exceptional examples of propagules with densities somewhere between seawater and freshwater18. Previous studies of the impacts of climate change on mangroves have focused on factors such as sea level rise, altered precipitation regimes and increasing temperature and storm frequency4,21,22,23 but the potential impact of climate-driven changes in seawater properties on mangroves has not yet been examined. This is somewhat surprising, as the ocean is the primary dispersal medium of this ‘sea-faring’ coastal vegetation and dispersal is a key process that governs a species’ response to climate change by changing its geographical range. This knowledge gap contrasts with recent efforts to expose links between climate change and dispersal in other ecologically important marine taxa such as zooplankton and fish species24,25,26,27.
In this study, we investigate predicted changes in sea surface temperature (SST), sea surface salinity (SSS) and sea surface density (SSD) for coastal waters bordering mangrove forests (hereafter referred to as ‘coastal mangrove waters’), over the next century. Using a biogeographic classification system for coastal and shelf areas28, we examine spatiotemporal changes in these surface ocean properties, with a particular focus on the world’s two major mangrove diversity hotspots: (1) the Atlantic East Pacific (AEP) region, including all of the Americas, West and Central Africa and (2) the Indo West Pacific (IWP) region, extending from East Africa eastwards to the islands of the central Pacific1. Finally, we synthesize available data on the density of mangrove propagules for different mangrove species and explore the potential impact of climate-driven changes in SSD on propagule dispersal.
To assess changes in SST and SSS throughout the global range of mangrove forests, we used present (2000–2014) and future (2090–2100) surface ocean properties from the Bio-ORACLE database29,30. SSD estimates were derived from these variables using the UNESCO EOS-80 equation of state polynomial for seawater31. Changes in SST, SSS and SSD (Fig. 1) were calculated for four representative concentration pathways (RCPs) and derived for coastal waters closest to the 583,578 polygon centroids from the 2015 Global Mangrove Watch (GMW) database32. After removing duplicates, our dataset contained 10,108 unique mangrove occurrence locations, with corresponding present conditions and predicted future changes in mean SST, SSS and SSD. Under the low-warming scenario RCP 2.6, mean SST of coastal mangrove waters is predicted to change by +0.64 (±0.11) °C and mean SSS by −0.06 (±0.25) practical salinity units (PSU). Combined, this results in an average change in mean SSD of −0.25 (±0.20) kg m−3 in coastal mangrove waters by the late twenty-first century (Supplementary Table 1). These values roughly double under RCP 4.5 (Supplementary Table 2), while under RCP 6.0, a change of +1.69 (±0.14) °C in mean SST, −0.21 (±0.42) PSU in mean SSS and −0.71 (±0.32) kg m−3 in mean SSD is predicted (Supplementary Table 3). Under RCP 8.5, our study predicts a change in SST of +2.84 (±0.21) °C (range 2.11–4.01 °C), a change in SSS of −0.30 (±0.74) PSU (−2.01–1.26 PSU) and a corresponding change in SSD of −1.17 (±0.56) kg m−3 (−2.53–0.03 kg m−3) (Supplementary Table 4).
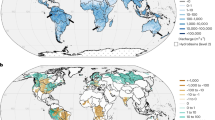
Spatial variability in predicted surface ocean property changes was examined by considering the two major mangrove bioregions (AEP and IWP) (Fig. 2) and using the Marine Ecoregions of the World (MEOW) biogeographic classification28 (Fig. 3). Both the range and changes in mean SST were comparable for the AEP and IWP mangrove bioregions, for all respective RCP scenarios (Fig. 2a and Supplementary Tables 1–4). Under RCP 8.5, mean SST in both mangrove bioregions is predicted to warm ~2.8 °C by 2100, which is roughly 4.5 times the predicted increase in mean SST under RCP 2.6 (Supplementary Tables 1 and 4). Predictions for the RCP 8.5 scenario are generally consistent with reported global ocean temperature trends33 and show that the greatest warming occurs in coastal waters near the Galapagos Islands (change in mean SST of 3.92 ± 0.06 °C). Pronounced SST increases are also predicted for Hawaii (change in mean SST of 3.36 ± 0.05 °C), the Southeast Australian Shelf (3.30 ± 0.25 °C), Northern and Southern New Zealand (3.25 ± 0.07 °C and 3.34 ± 0.02 °C, respectively), Warm Temperate Northwest Pacific (3.27 ± 0.16 °C), the Red Sea and Gulf of Aden (3.24 ± 0.08 °C), Somali/Arabian Coast (3.23 ± 0.15 °C), South China Sea (3.07 ± 0.10 °C), the Tropical East Pacific (3.09 ± 0.15 °C) and the Warm Temperate Northwest Atlantic (3.14 ± 0.13 °C) (Fig. 3b and Supplementary Tables 4).
a–c, Variation in SST (a), SSS (b) and SSD (c) under various RCP scenarios. Grey indicates global distribution (n = 10,108), orange denotes AEP (n = 3,190) and green represents IWP (n = 6,918). Data for SST and SSS consist of present-day (2000–2014) and future (2090–2100) marine fields from the Bio-ORACLE database29,30, from which SSD data were derived. The cat-eye plots50 show the distribution of the data. Median and mean values are indicated with black and white circles, respectively, and the vertical lines represent the interquartile range.
a, Global map showing the provinces (colour code and numbers) from the MEOW database28 used to investigate spatial patterns in mangrove coastal ocean water changes by 2100. b–d, Longitudinal gradient of the change in SST (b), SSS (c) and SSD (d) under RCP 8.5 in the AEP and the IWP mangrove bioregions; circles are coloured according to the MEOW province in which respective mangrove sites are located.
Predicted SSS changes exhibit an opposite trend in the AEP and IWP bioregions, with increased salinity in the AEP and reduced salinity in the IWP under global warming (RCP 2.6–RCP 8.5; Fig. 2b); this is reflected in contrasting SSD changes in both mangrove bioregions (Fig. 2c) and associated with predicted global changes in precipitation, with extensions of the rainy season over most of the monsoon domains, except for the American monsoon34. Under RCP 8.5, the spatially averaged change in mean SSS is +0.51 (±0.57) PSU in the AEP and −0.68 (±0.44) PSU in the IWP region. The maximum decrease in mean SSS (−2.01 PSU) is predicted for the Gulf of Guinea in the AEP bioregion (Fig. 3c and Supplementary Table 4). Within the IWP, the Western Indian Ocean region shows little or no changes in SSS, which contrasts with the pronounced freshening trends predicted in the eastern part of this ocean basin and the Tropical West Pacific (Figs. 1b and 3c). Increased freshening is predicted in the Bay of Bengal (SSS change: −1.17 ± 0.43 PSU), the Sunda Shelf (SSS change: −1.21 ± 0.29 PSU) and the Western Coral Triangle province (mean SSS change: −0.80 ± 0.17 PSU) (Fig. 3c and Supplementary Table 4). Within the AEP, salinity increases exceed +0.96 PSU in the Tropical Northwestern Atlantic, +0.80 in the Warm Temperate Northwest Atlantic and +0.68 in the West African Transition (Fig. 3c and Supplementary Table 4). The spatial heterogeneity in SSS across the global range of mangrove forests corresponds with observed changes in SSS35. Trends in SSD (Fig. 3d) strongly track changes in SSS (Fig. 3c) rather than SST. All RCP scenarios predict an overall decrease in SSD for both mangrove bioregions; however, the predicted decrease in SSD in the IWP region was a factor of 2 (RCP 6.0) and 2.5 (RCP 2.6, RCP 4.5 and RCP 8.5) stronger than in the AEP (Figs. 2 and 3d and Supplementary Tables 1–4).
Propagule density values from our literature survey range from <600 kg m−3 to >1,080 kg m−3 for different mangrove species (Fig. 4 and Supplementary Table 5). The low densities reported for Heritiera littoralis propagules provide a strong contrast with the near-seawater propagule densities reported for Avicennia and members of the Rhizophoraceae (Bruguiera, Rhizophora and Ceriops). Floating characteristics of the latter may be particularly sensitive to changes in SSD. To illustrate the potential influence of changing ocean conditions on mangrove propagule dispersal, we considered threshold water density values (1,020 and 1,022 kg m−3) that are within the range where elongated propagules of important mangrove genera tend to change floating orientation (Fig. 4a). More specifically, we determined the ocean surface area with an SSD below or equal to these thresholds under different climate change scenarios (Fig. 5). Under RCP 8.5, the ocean surface covered by mangrove coastal waters (coastal waters bordering present mangrove forests) with a density ≤1,020 kg m−3 increases ~27% by 2100, notably more so in the IWP (~37%) than in the AEP (~6%) (Supplementary Table 6). A threshold of 1,022 kg m−3 results in increases of roughly +11% (global), +12% (IWP) and +8% (AEP) (Supplementary Table 7). Similar spatial patterns are observed for open-ocean waters within the global latitudinal range of mangroves (Fig. 5 and Supplementary Figs. 1 and 2).
a, Range of reported propagule density values for wide-ranging mangrove species and present and future range of SSD for coastal waters along the range of those mangrove species. Mangrove propagule data are extracted from the literature (Supplementary Table 5). H. lit, Heritiera littoralis; X. gra, Xylocarpus granatum; A. ger, Avicennia germinans; A. mar, Avicennia marina; B. gym, Bruguiera gymnorrhiza; C. tag, Ceriops tagal; R. man, Rhizophora mangle; R. muc, Rhizophora mucronata. Bottom part adapted from ref. 51. b, Conceptual figure of the potential effects of ocean warming and freshening on mangrove propagule dispersal. Ocean warming and freshening drive changes in SSD and may reduce the timeframe for opportunistic colonization. For a propagule with a specific density and floating profile under present surface ocean conditions, reduced SSD of coastal and open-ocean waters may reduce floatation time (shaded area) and hence, reduce the proportion of long-distance dispersers. For simplicity, the density of propagules is assumed to increase linearly over time, although the actual increase may be nonlinear.
a–d, Spatial extent of coastal and open-ocean surface waters with a density ≤1,020 kg m−3 (a,b) and 1,022 kg m−3 (c,d), for present (2000–2014) (a,c) and future (2090–2100; RCP 8.5) (b,d) scenarios. Data are shown for surface ocean waters within the global latitudinal range of mangrove forests (between 32° N and 38° S). The two density thresholds considered are within the range of densities at which mangrove propagule buoyancy and floating orientation of several mangrove genera change, as reported in available literature. Black dots along the coast represent the global mangrove extent from the 2015 GMW dataset32. Magenta-coloured circles represent SSD values <1,014 kg m−3. e,f, Ocean area with a density less than or equal to SSD (e) and future changes in the spatial extent of these regions (f) for different RCP scenarios.
Our study shows changes in the physical and chemical properties of coastal mangrove waters by the end of the twenty-first century that could affect the distribution of propagules from widespread mangrove genera (Avicennia, Bruguiera, Ceriops and Rhizophora) and probably more so within the IWP region, the primary hotspot of mangrove diversity, compared to the AEP. Propagules from these genera have densities that are close to that of seawater and experimental evidence shows that propagules in species of these genera typically become denser as they age36,37,38,39. For mangroves in large parts of the IWP, as well as the Gulf of Guinea in the AEP, declines in SSD could therefore promote local sinking rates and reduce the probability of successful long-distance dispersal due to earlier propagule sinking before reaching suitable establishment zones (Fig. 4). Indeed, future changes in surface ocean properties may impact dispersal differently at different scales. More specifically, short-distance dispersal may be promoted and successful long-distance dispersal reduced since (1) freshening of local waters near the propagule release site and associated sinking can reduce the fraction of propagules reaching open-ocean waters; and (2) older propagules that have travelled longer distances are at risk of offshore sinking due to the combined effect of increased propagule density during transport36,37,38,39 and fresher coastal waters; as such, increased ocean freshening and warming and resulting changes in SSD are likely to reduce the tail of the dispersal kernel—which captures long-distance dispersal events. Upon sinking, a propagule is at least temporarily eliminated from the dispersing cohort and prone to mortality associated with stranding in unsuitable conditions. Empirical evidence of mangrove distributions limited by sinking of propagules under increasingly fresher conditions in estuaries was shown for mangroves along the Nakara River in Japan36. Such effects are of considerable importance, since some of the most pronounced changes in SSS and SSD are near major river outlets where many of the most extensive mangrove areas in the world are found1, such as the Ganges–Brahmaputra delta. In contrast to the wide-ranging mangrove genera Avicennia, Bruguiera, Ceriops and Rhizophora, mangrove species such as H. littoralis and Xylocarpus granatum are unlikely to be affected by these ocean changes as their propagules possess very low densities13.
An additional level of complexity is that the floating orientation of propagules from several widespread mangrove genera (Rhizophora and Ceriops) could move between horizontal and vertical due to small changes in density38,40,41. Changes in floating orientation have been associated with the replacement of air in intercellular tissue by water via lenticels38; however, while the exact anatomical and physiological mechanisms underlying changes in mangrove propagule buoyancy are not yet fully understood, experimental and modelling studies showed that these changes in floating orientation can strongly alter dispersal trajectories at the landscape (102–103 m), regional (103–105 m), as well as the biogeographic scale (105–107 m), via the relative effects of ocean and wind forces on propagule transport42,43,44.
Besides effects of changes in SSD, propagule dispersal may also be directly impacted by SSS or SST. In Avicennia marina, propagule sinking has been associated with the shedding of the pericarp (Steinke, 1975, as cited in ref. 45) and the time required for pericarp shedding and the separation of the cotyledons increases with increasing salinity (Downton, 1982, as cited in ref. 45). As such, future ocean freshening might decrease floating periods and potentially dispersal distances in this species. Increases in SSS might also result in higher propagule mortality rates and lower germination success40, whereas lower SSS could reduce propagule viability by increasing the incidence of fungal infestation and rotting46. Finally, projected increases in SST may facilitate mangrove expansion to higher latitudes in some regions by reducing the negative effect of colder oceanic waters on propagule viability47. For example, an earlier study on the western South Atlantic latitudinal mangrove range limit reported that temperatures <20 °C may limit the viability of mangrove propagules during their dispersal along this coast and during subsequent establishment48. However, since empirical data on potential direct effects of SSS and SST on mangrove propagule dispersal are deficient, effects of these variables require further investigation.
It is important to note that changes indicated by our study are based on changes in time-mean surface ocean properties and that the actual variability in SSD around these mean values could be higher. Since mangroves thrive in a broad range of coastal settings, including estuaries, deltas, lagoons and open coast, their propagules already encounter a wide range of water densities today but our findings clearly illustrate that for important mangrove regions worldwide exposure to lower-density waters will occur more frequently in the future. While our results suggest changes in dispersal patterns following climate-driven changes in coastal and open-ocean surface-water properties, the effects also depend on adaptive capacity. Under current ocean conditions, propagule densities that are just slightly lower than that of seawater are probably adaptive, since this ensures that they sink in favourable coastal environments, that is, along coastal zones where SSS and SSD are lower due to freshwater influx from rivers. Yet, in future oceans the spatial extent of these lower-density waters may expand further offshore, altering buoyancy characteristics and the spatial distribution of propagules. The demographic costs associated with ending up in unsuitable habitat (for example, due to offshore sinking) can get balanced when populations evolve to have lower propagule densities. As such, there is a need for quantitative data for inter- and intra-population variation in the critical SSD at which mangrove propagules sink, which could reflect underlying genetic variation in propagule density that can fuel evolutionary change. Overall, our results suggest that we may be entering a density-induced transition phase as part of the Anthropocene, highlighting the importance of considering future ocean property changes in evaluating the impacts of climate change on mangrove ecosystems. Such information will complement knowledge on the effects of other impacting factors and is important for predicting how altered environmental conditions will affect these sensitive forests. Finally, while we considered mangroves as a model system in this study, our findings may be relevant also for other coastal taxa producing sea-drifted propagules, such as seagrasses49 and coastal strand communities (Terminalia catappa, Barringtonia asiatica, Thespesia populnea, Hibiscus tiliaceus, Pisonia grandis, Pandanus spp. and so on).
Methods
Ocean data
In this study we examine changes in SST, SSS and SSD over the next century. SSD has been largely overlooked in studies that examine the response of marine organisms to climate-driven ocean changes, despite its potential influence on the dispersal patterns and colonization potential of passive propagules with near-seawater densities. We calculated changes in mean SST and SSS over the next century using present (2000–2014) and future (2090–2100) data from Bio-ORACLE29,30, a database of GIS rasters providing geophysical, biotic and environmental data for surface and benthic marine realms (https://www.bio-oracle.org/). These data consist of uniformly constructed rasters provided at a spatial resolution of 5 arcmin (~0.08° or ~9.2 km at the Equator). Present layers were generated with climate data describing monthly means for the period 2000–2014, acquired from preprocessed global ocean ARMOR reanalyses that combine remotely sensed and in situ observations, while future data were produced by averaging output from atmosphere–ocean general circulation models provided by the Coupled Model Intercomparison Project Phase 5 (CMIP5)30. We are aware of the limitations of remotely sensed coastal ocean data and climate change projections of fine-scale near-shore circulation patterns, which may deviate from in situ observations52. However, using time-averaged SST and SSS data and considering spatially averaged patterns ensures that we are capturing general trends in coastal ocean changes. SSD was derived from these variables using the UNESCO EOS-80 equation of state polynomial for seawater31. Changes were calculated for four RCP scenarios: RCP 2.6 (490 CO2e before 2100 and then decline), RCP 4.5 (650 CO2e at stabilization after 2100), RCP 6.0 (850 CO2e at stabilization after 2100) and RCP 8.5 (>1,370 CO2e in 2100) (see ref. 53 for more details about RCPs).
Global mangrove range data
The GMW provides high-resolution (0.8 arcsec or ~25 m) global mangrove extent baseline maps based on Landsat sensor spectral composite data and Advanced Land Observing Satellite (ALOS) Phased Arrayed L-band Synthetic Aperture Radar (PALSAR) data for the years 1996, 2007, 2008, 2009, 2010, 2015 and 2016 (ref. 32). For this study, we used the 2015 mangrove extent baseline map since it most closely matches the end year of the period (2000–2014) considered for generating the Bio-ORACLE raster data of present-day marine environmental conditions30. Using the QGIS 3.10.10 software, centroids were computed for each GMW 2015 polygon (n = 583,578) and considered as our global mangrove occurrence dataset. Global mangrove occurrence points were assigned to the nearest grid cell (Euclidean distance) in the Bio-ORACLE fields. Since multiple occurrence points might have been assigned to the same grid cell, duplicates were removed, resulting in unique grid cell records for the GMW 2015 mangrove range (n = 10,108). Each of these steps was performed using the MATLAB 2020b programming software.
Mangrove species-specific data
A literature survey was conducted to collect data on mangrove propagule density values that were interpreted against the range of present and future SSD values within the range of Avicennia germinans (AEP), Avicennia marina (IWP), Bruguiera gymnorrhiza (IWP), Ceriops tagal (IWP), Heritiera littoralis (IWP), Rhizophora mangle (AEP), Rhizophora mucronata (IWP) and Xylocarpus granatum (IWP). This selection of mangrove species in our literature survey is based on the availability of propagule density data. Such data are surprisingly limited but nevertheless allow for a first assessment of species-specific differences in the sensitivity to SSD changes. Additionally, propagules of the selected species are representative for the variety of propagule morphotypes found among mangrove species globally and include the most widely distributed mangrove species (Avicennia spp. and Rhizophora spp.) for which distributional changes have been reported and predicted5,54,55. In the case that specific gravity values were reported36,56, we multiplied these values by 999.97 kg m−3 (the density of water at 4 °C, which is the standard density used to compute specific gravity) to obtain density values.
The 2015 GMW database used for our global analysis does not contain coordinates for species-specific mangrove distributions. Hence, data for different species were generated using vector layers obtained from the IUCN Red List website (https://www.iucnredlist.org/). The IUCN data for each species consist of a single polygon feature that was intersected with a 1:10 m global coastline downloaded from the Natural Earth database (https://www.naturalearthdata.com/) in QGIS 3.10.10. Before this intersection, we buffered the species distribution polygons using a buffer distance equal to the spatial resolution of the Bio-ORACLE marine data layers (0.5 arcmin) to avoid losing data where the original IUCN species distribution polygons did not overlap with the global coastline data. Point features were generated at 1 km distance for each line feature representing a species range, using the QChainage plugin in QGIS 3.10.10 and longitude and latitude information was added to each point for A. germinans (n = 4,610), A. marina (n = 11,231), B. gymnorrhiza (n = 9,665), C. tagal (n = 9,134), H. littoralis (n = 7,984), R. mangle (n = 3,431), R. mucronata (n = 9,519) and X. granatum (n = 8,890). Since the IUCN data show mangrove presence along vast stretches of coast (for example, Somalia and East Madagascar) where mangroves are absent in the 2015 GMW data, we extracted ocean data for the IUCN coordinates that correspond with a 2015 GMW occurrence point (the centroids of each 2015 GMW polygon feature). The resulting coordinates for A. germinans (n = 2,964), A. marina (n = 6,419), B. gymnorrhiza (n = 6,040), C. tagal (n = 5,653), H. littoralis (n = 5,100), R. mangle (n = 2,345), R. mucronata (n = 5,348) and X. granatum (n = 5,891) can be viewed as species-specific GMW data. Each of these computations was performed using the MATLAB 2020b programming software.
Spatial analyses
Spatial patterns in projected ocean property changes over the next century were explored by considering bioregion- and province-levels to subset our GMW 2015 data. Bioregion subsetting consists of coastal Bio-ORACLE cells in the AEP (between −180° and 19° longitude) and IWP (between 19° and 180° longitude) bioregion, whereas the province-level waters consist of a subsetting using the provinces provided by the MEOW dataset28.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All the datasets used for analyses during this study are publicly available and can be accessed at: https://www.bio-oracle.org/ (marine data layers29,30); https://data.unep-wcmc.org/datasets/45 (global mangrove extent32); https://www.iucnredlist.org/ (geographic range of the species considered); https://data.unep-wcmc.org/datasets/38 (provinces from the MEOW database28). All remaining data that support the findings of this study are freely available at https://doi.org/10.5061/dryad.66t1g1k4c (ref. 57).
Code availability
Computations of SSD were conducted using the UNESCO EOS-80 equation of state polynomial for seawater (sw_dens.m from the MATLAB seawater package)31. Other MATLAB codes used during the current study are available from the corresponding author upon reasonable request.
References
Spalding, M., Kainuma, M. & Collins, L. World Atlas of Mangroves (Earthscan and James & James, 2010).
Barbier, E. B. et al. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81, 169–193 (2011).
Ellison, J. Vulnerability assessment of mangroves to climate change and sea-level rise impacts. Wetl. Ecol. Manag. 23, 115–137 (2015).
Ward, R. D., Friess, D. A., Day, R. H. & MacKenzie, R. A. Impacts of climate change on mangrove ecosystems: a region by region overview. Ecosyst. Health Sust. 2, e01211 (2016).
Saintilan, N. et al. Mangrove expansion and salt marsh decline at mangrove poleward limits. Glob. Change Biol. 20, 147–157 (2014).
Cavanaugh, K. C. et al. Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events. Proc. Natl Acad. Sci. USA 111, 723–727 (2014).
Kelleway, J. J. et al. Review of the ecosystem service implications of mangrove encroachment into salt marshes. Glob. Change Biol. 23, 3967–3983 (2017).
Pecl, T. P. et al. Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355, 1389 (2017).
Osland, M. J. et al. Climatic controls on the global distribution, abundance, and species richness of mangrove forests. Ecol. Monogr. 87, 341–359 (2017).
Thom, B. G. Mangrove ecology and deltaic geomorphology: Tabasco, Mexico. J. Ecol. 55, 301–343 (1967).
Duke, N. C., Ball, M. C. & Ellison, J. C. Factors influencing biodiversity and distributional gradients in mangroves. Glob. Ecol. Biogeogr. Lett. 7, 27–47 (1998).
Raw, J. L., Godbold, J. A., van Niekerk, L. & Adams, J. B. Drivers of mangrove distribution at the high-energy, wave-dominated, southern African range limit. Estuar. Coast. Shelf Sci. 226, 106296 (2019).
Van der Stocken, T. et al. A general framework for propagule dispersal in mangroves. Biol. Rev. 94, 1547–1575 (2019).
Cavanaugh, K. C. et al. Climate-driven regime shifts in a mangrove–salt marsh ecotone over the past 250 years. Proc. Natl Acad. Sci. USA 116, 21602–21608 (2019).
Woodroffe, C. D. & Grindrod, J. Mangrove biogeography: the role of Quaternary environmental and sea-level change. J. Biogeogr. 18, 479–492 (1991).
Hoppe-Speer, S. C. L., Adams, J. B. & Rajkaran, A. Mangrove expansion and population structure at a planted site, East London, South Africa. South. For. 77, 131–139 (2015).
Van der Stocken, T. et al. Global-scale dispersal and connectivity in mangroves. Proc. Natl Acad. Sci. USA 116, 915–922 (2019).
Guppy, H. B. Observations of a Naturalist in the Pacific Between 1896 and 1899: Plant Dispersal Vol. II (Macmillan and Co., 1906).
Bindoff, N. L. et al. in Special Report on the Ocean and Cryosphere in a Changing Climate (eds Pörtner, H.-O. et al.) 447–587 (WMO, 2019).
Johnson, G. C. & Wijffels, S. E. Ocean density change contributions to sea level rise. Oceanography 24, 112–121 (2011).
Alongi, D. M. The impact of climate change on mangrove forests. Curr. Clim. Change Rep. 1, 30–39 (2015).
Lovelock, E. C. et al. The vulnerability of Indo-Pacific mangrove forests to sea-level rise. Nature 526, 559–563 (2015).
Osland, M. J. et al. Mangrove forests in a rapidly changing world: global change impacts and conservation opportunities along the Gulf of Mexico coast. Estuar. Coast. Shelf Sci. 214, 120–140 (2018).
O’Connor, M. I. et al. Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc. Natl Acad. Sci. USA 104, 1266–1271 (2007).
Wilson, L. J. et al. Climate-driven changes to ocean circulation and their inferred impacts on marine dispersal patterns. Glob. Ecol. Evol. 25, 923–939 (2016).
Poloczanska, E. S. et al. Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 3, 62 (2016).
Pinsky, M. L., Eikeset, A. M., McCauley, D. J., Payne, J. L. & Sunday, J. M. Greater vulnerability to warming of marine versus terrestrial ectotherms. Nature 569, 108–111 (2019).
Spalding, M. D. et al. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience 57, 573–583 (2007).
Tyberghein, L. et al. Bio-ORACLE: a global environmental dataset for marine species distribution modelling. Glob. Ecol. Biogeogr. 21, 272–281 (2012).
Assis, J. et al. Bio-ORACLE v2.0: extending marine data layers for bioclimatic modelling. Glob. Ecol. Biogeogr. 27, 277–284 (2017).
Fofonoff, P. & Millard, R. C. Algorithms for computation of fundamental properties of seawater. UNESCO Techn. Papers in Mar. Sci. 44 (UNESCO, 1983).
Bunting, P. et al. The Global Mangrove Watch—a new 2010 global baseline of mangrove extent. Remote Sens. 10, 1669 (2018).
Bruno, J. F. et al. Climate change threatens the world’s marine protected areas. Nat. Clim. Change 8, 499–503 (2018).
Moon, S. & Ha, K. J. Future changes in monsoon duration and precipitation using CMIP6. Clim. Atmos. Sci. 3, 45 (2020).
Durack, P. J. & Wijffels, S. E. Fifty-year trends in global ocean salinities and their relationship to broad-scale warming. J. Clim. 23, 4342–4362 (2010).
Kadoya, T. & Inoue, T. Spatio-temporal pattern of specific gravity of mangrove diaspore: implications for upstream dispersal. Ecography 38, 472–479 (2015).
Robert, E. M. R. et al. Viviparous mangrove propagules of Ceriops tagal and Rhizophora mucronata, where both Rhizophoraceae show different dispersal and establishment strategies. J. Exp. Mar. Bio. Ecol. 468, 45–54 (2015).
Tonné, N., Beeckman, H., Robert, E. M. R. & Koedam, N. Towards an unknown fate: the floating behaviour of recently abscised propagules from wide ranging Rhizophoraceae mangrove species. Aquat. Bot. 140, 23–33 (2017).
Wang, W., Li, X. & Wang, M. Propagule dispersal determines mangrove zonation at intertidal and estuarine scales. Forests 10, 245 (2019).
Rabinowitz, D. Dispersal properties of mangrove propagules. Biotropica 10, 47–57 (1978).
Clarke, P. J., Kerrigan, R. A. & Westphal, C. J. Dispersal potential and early growth in 14 tropical mangroves: do early life history traits correlate with patterns of adult distribution? J. Ecol. 89, 648–659 (2001).
Van der Stocken, T. et al. The role of wind in hydrochorous mangrove propagule dispersal. Biogeosciences 10, 3635–3647 (2013).
Van der Stocken, T. et al. Interaction between water and wind as a driver of passive dispersal in mangroves. PLoS ONE 10, e0121593 (2015).
Van der Stocken, T. & Menemenlis, D. Modelling mangrove propagule dispersal trajectories using high-resolution estimates of ocean surface winds and currents. Biotropica 49, 472–481 (2017).
Hutchings, P. & Saenger, P. The Ecology of Mangroves (Univ. of Queensland Press, 1987).
Alleman, L. K. & Hester, M. W. Reproductive ecology of black mangrove (Avicennia germinans) along the Louisiana coast: propagule production cycles, dispersal limitations, and establishment elevations. Estuar. Coast. 34, 1068–1077 (2011).
Hickey, M. H. et al. Is climate change shifting the poleward limit of mangroves?. Estuar. Coast 40, 1215–1226 (2017).
Soares, M. L. G., Estrada, G. C. D. E., Fernandez, V. & Tognella, M. M. P. Southern limit of the western South Atlantic mangroves: assessment of the potential effects of global warming from a biogeographical perspective. Estuar. Coast. Shelf Sci. 101, 44–53 (2012).
Repolho, T. et al. Seagrass ecophysiological performance under ocean warming and acidification. Sci. Rep. 7, 41443 (2017).
Allen, M. et al. Raincloud plots: a multi-platform tool for robust data visualization (version 2). Wellcome Open Res. 4, 63 (2021).
Van der Stocken, T., Vanschoenwinkel, B., De Ryck, D. & Koedam, N. Caught in transit: offshore interception of seafaring propagules from seven mangrove species. Ecosphere 9, e02208 (2018).
Smit, J. S. et al. A coastal seawater temperature dataset for biogeographic studies: large biases between in situ and remotely-sensed data sets around the coast of South Africa. PLOS ONE 8, e81944 (2013).
Moss, R. et al. The next generation of scenarios for climate change research and assessment. Nature 463, 747–756 (2010).
Quisthoudt, K. et al. Disentangling the effects of global climate and regional land-use change on the current and future distribution of mangroves in South Africa. Biodivers. Conserv. 22, 1369–1390 (2013).
Cavanaugh, K. C. et al. Sensitivity of mangrove range limits to climate variability. Glob. Ecol. Biogeogr. 27, 925–935 (2018).
McKee, L. K. Seedling recruitment patterns in a Belizean mangrove forest: effects of establishment ability and physico-chemical factors. Oecologia 101, 448–460 (1995).
Van der Stocken, T. et al. Future changes in mangrove coastal water properties. Dryad https://doi.org/10.5061/dryad.66t1g1k4c (2022).
Acknowledgements
T.V.d.S. is supported by the EU Horizon 2020 Framework Programme for Research and Innovation under the Marie Skłodowska-Curie actions Individual Fellowship (MSCA-IF) with grant agreement no. 896888 (GLOMAC). K.C.C. is supported by the NASA Land Cover and Land Use Change (LCLUC) Program with grant no. 80NSSC21K0296.
Author information
Authors and Affiliations
Contributions
T.V.d.S. designed the study, compiled and analysed the data, produced the figures, interpreted the results and wrote the manuscript with contributions and suggestions from B.V., D.C., K.C. and N.K. T.V.d.S and D.C. computed sea surface density fields. B.V. provided extensive feedback on various drafts and revisions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Climate Change thanks Ken Krauss, Erik Yando and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–7 and Figs. 1 and 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Van der Stocken, T., Vanschoenwinkel, B., Carroll, D. et al. Mangrove dispersal disrupted by projected changes in global seawater density. Nat. Clim. Chang. 12, 685–691 (2022). https://doi.org/10.1038/s41558-022-01391-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-022-01391-9
This article is cited by
-
Global habitat suitability modeling reveals insufficient habitat protection for mangrove crabs
Scientific Reports (2022)