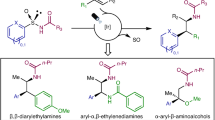
Abstract
Arylethylamines are popular structural elements in bioactive molecules but are often made through a linear series of synthetic steps. A modular protocol to assemble arylethylamines from alkenes in one step would represent a useful advance in discovery chemistry, though current limitations preclude a generally applicable method. In this work we disclose an aminoarylation of alkenes using aryl sulfinamide reagents as bifunctional amine and arene donors. This reaction features excellent regioselectivity and diastereoselectivity on a variety of activated and unactivated substrates. Using a weakly oxidizing photocatalyst, a nitrogen radical is generated under mild conditions and adds to an alkene to form a new C–N bond. A desulfinylative aryl migration event known as a Smiles–Truce rearrangement follows to form a new C–C bond. In this manner, arylethylamines can be rapidly assembled from abundant alkene feedstocks. Moreover, chiral information from the sulfinamide can be transferred via rearrangement to a new carbon stereocentre in the product, thus advancing the development of traceless asymmetric alkene difunctionalization.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data in support of the findings of this study are available within the Article and its Supplementary Information. X-ray crystallographic data for compound 2u are freely available from the Cambridge Crystallographic Data Center (CCDC 2150974).
References
Mieda, R. et al. Comparison of four documents describing adrenaline purification, and the work of three important scientists, Keizo Uenaka, Nagai Nagayoshi and Jokichi Takamine. J. Anesth. Hist. 6, 42–48 (2020).
Gainetdinov, R. R., Hoener, M. C. & Berry, M. D. Trace amines and their receptors. Pharmacol. Rev. 70, 549–620 (2018).
Sherwani, S. I. & Khan, H. A. in Trace Amines and Neurological Disorders (eds Farooqui, T. & Farooqui, A. A.) 269–284 (Academic Press, 2016).
Wu, R., Liu, J. & Li, J.-X. in Advances in Pharmacology Vol. 93 (ed. Li, J.-X.) 373–401 (Academic Press, 2022).
Grace, A. A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 17, 524–532 (2016).
Hollopeter, G. et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409, 202–207 (2001).
Jones, P., Wilcoxen, K., Rowley, M. & Toniatti, C. Niraparib: a poly(ADP-ribose) polymerase (PARP) inhibitor for the treatment of tumors with defective homologous recombination. J. Med. Chem. 58, 3302–3314 (2015).
Ryu, J.-S., Li, G. Y. & Marks, T. J. Organolathanide-catalyzed regioselective intermolecular hydroamination of alkenes, alkynes, vinylarenes, di- and trivinylarenes, and methylenecyclopropanes. Scope and mechanistic comparison to intramolecular cyclohydroaminations. J. Am. Chem. Soc. 125, 12584–12605 (2003).
Utsunomiya, M. & Hartwig, J. F. Ruthenium-catalyzed anti-Markovnikov hydroamination of vinylarenes. J. Am. Chem. Soc. 126, 2702–2703 (2004).
Nielsen, D. K., Huang, C.-Y. & Doyle, A. G. Directed nickel-catalyzed Negishi cross coupling of alkyl aziridines. J. Am. Chem. Soc. 135, 13605–13609 (2013).
Duda, M. L. & Michael, F. E. Palladium-catalyzed cross-coupling of N-sulfonylaziridines with boronic acids. J. Am. Chem. Soc. 135, 18347–18349 (2013).
Steiman, T. J., Liu, J., Mengiste, A. & Doyle, A. G. Synthesis of β-phenethylamines via Ni/photoredox cross-electrophile coupling of aliphatic aziridines and aryl iodides. J. Am. Chem. Soc. 142, 7598–7605 (2020).
Lippa, R. A., Battersby, D. J., Murphy, J. A. & Barrett, T. N. Synthesis of arylethylamines via C(sp3)–C(sp3) palladium-catalyzed cross-coupling. J. Org. Chem. 86, 3583–3604 (2021).
Boyington, A. J., Seath, C. P., Zearfoss, A. M., Xu, Z. & Jui, N. T. Catalytic strategy for regioselective arylethylamine synthesis. J. Am. Chem. Soc. 141, 4147–4153 (2019).
Wang, D. et al. Asymmetric copper-catalyzed intermolecular aminoarylation of styrenes: efficient access to optical 2,2-diarylethylamines. J. Am. Chem. Soc. 139, 6811–6814 (2017).
Jiang, H., Yu, X., Daniliuc, C. G. & Studer, A. Three-component aminoarylation of electron-rich alkenes by merging photoredox with nickel catalysis. Angew. Chem. Int. Ed. 60, 14399–14404 (2021).
Margrey, K. A. & Nicewicz, D. A. A general approach to catalytic alkene anti-Markovnikov hydrofunctionalization reactions via acridinium photoredox catalysis. Acc. Chem. Res. 49, 1997–2006 (2016).
Kang, T. et al. Nickel-catalyzed 1,2-carboamination of alkenyl alcohols. J. Am. Chem. Soc. 143, 13962–13970 (2021).
Liu, Z. et al. Catalytic intermolecular carboamination of unactivated alkenes via directed aminopalladation. J. Am. Chem. Soc. 139, 11261–11270 (2017).
Kang, T. et al. Alkene difunctionalization directed by free amines: diamine synthesis via nickel-catalyzed 1,2-carboamination. ACS Catal. 12, 3890–3896 (2022).
Zeng, W. & Chemler, S. R. Copper(II)-catalyzed enantioselective intramolecular carboamination of alkenes. J. Am. Chem. Soc. 129, 12948–12949 (2007).
Wolfe, J. P. Palladium-catalyzed carboetherification and carboamination reactions of γ-hydroxy- and γ-aminoalkenes for the synthesis of tetrahydrofurans and pyrrolidines. Eur. J. Org. Chem. 2007, 571–582 (2007).
Zheng, S., Gutierrez-Bonet, A. & Molander, G. A. Merging photoredox PCET with nickel-catalyzed cross-coupling: cascade amidoarylation of unactivated olefins. Chem 5, 339–352 (2019).
Angelini, L. et al. Reaction of nitrogen-radicals with organometallics under Ni-catalysis: N-arylations and amino-functionalization cascades. Angew. Chem. Int. Ed. 58, 5003–5007 (2019).
Brenzovich, W. E. Jr. et al. Gold-catalyzed intramolecular aminoarylation of alkenes: C—C bond formation through bimolecular reductive elimination. Angew. Chem. Int. Ed. 49, 5519–5522 (2010).
Tu, J.-L., Tang, W. & Liu, F. Photoredox-neutral alkene aminoarylation for the synthesis of 1,4,5,6-tetrahydropyridazines. Org. Chem. Front. 8, 3712–3717 (2021).
Bunescu, A., Abdelhamid, Y. & Gaunt, M. J. Multicomponent alkene azidoarylation by anion-mediated dual catalysis. Nature 598, 597–603 (2021).
Cai, Y., Chatterjee, S. & Ritter, T. Photoinduced copper-catalyzed late-stage azidoarylation of alkenes via arylthianthrenium salts. J. Am. Chem. Soc. 145, 13542–13548 (2023).
Truce, W. E., Ray, W. J., Norman, O. L. & Eickemeyer, D. B. Rearrangements of aryl sulfones. I. The metalation and rearrangement of mesityl phenyl sulfone1. J. Am. Chem. Soc. 80, 3625–3629 (1958).
Allen, A. R., Noten, E. A. & Stephenson, C. R. J. Aryl transfer strategies mediated by photoinduced electron transfer. Chem. Rev. 122, 2695–2751 (2022).
Whalley, D. M. & Greaney, M. F. Recent advances in the Smiles rearrangement: new opportunities for arylation. Synthesis 54, 1908–1918 (2022).
Henderson, A. R. P., Kosowan, J. R. & Wood, T. E. The Truce–Smiles rearrangement and related reactions: a review. Can. J. Chem. 95, 483–504 (2017).
Holden, C. M. & Greaney, M. F. Modern aspects of the Smiles rearrangement. Chem. Eur. J. 23, 8992–9008 (2017).
Loven, R. & Speckamp, W. N. A novel 1,4 arylradical rearrangement. Tetrahedron 13, 1567–1570 (1972).
Motherwell, W. B. & Pennell, A. M. K. A novel route to biaryls via intramolecular free radical ipso substitution reactions. J. Chem. Soc. Chem. Commun. https://doi.org/10.1039/C39910000877 (1991).
Tada, M., Shijima, H. & Nakamura, M. Smiles-type free radical rearrangement of aromatic sulfonates and sulfonamides: syntheses of arylethanols and arylethylamines. Org. Biomol. Chem. 1, 2499–2505 (2003).
Monos, T. M., McAtee, R. C. & Stephenson, C. R. J. Arylsulfonylacetamides as bifunctional reagents for alkene aminoarylation. Science 361, 1369–1373 (2018).
Allen, A. R. et al. Mechanism of visible light-mediated alkene aminoarylation with arylsulfonylacetamides. ACS Catal. 12, 8511–8526 (2022).
McAtee, R. C., Noten, E. A. & Stephenson, C. R. J. Arene dearomatization through a catalytic N-centered radical cascade reaction. Nat. Commun. 11, 2528 (2020).
Noten, E. A., McAtee, R. C. & Stephenson, C. R. J. Catalytic intramolecular aminoarylation of unactivated alkenes with aryl sulfonamides. Chem. Sci. 13, 6942–6949 (2022).
Irikura, K. K. & Todua, N. G. Facile Smiles-type rearrangement in radical cations of N-acyl arylsulfonamides and analogs. Rapid Commun. Mass Spectrom. 28, 829–834 (2014).
Liang, Y., Simón-Manso, Y., Neta, P., Yang, X. & Stein, S. E. CID fragmentation of deprotonated N-acyl aromatic sulfonamides. Smiles-type and nitrogen–oxygen rearrangements. J. Am. Soc. Mass. Spectrom. 32, 806–814 (2021).
Matos, P. M., Lewis, W., Moore, J. C. & Stockman, R. A. Sulfonimidates: useful synthetic intermediates for sulfoximine synthesis via C–S bond formation. Org. Lett. 20, 3674–3677 (2018).
Hervieu, C. et al. Asymmetric, visible light-mediated radical sulfinyl-Smiles rearrangement to access all-carbon quaternary stereocentres. Nat. Chem. 13, 327–334 (2021).
Tian, Q., He, P. & Kuang, C. Copper-catalyzed arylsulfonylation of N-arylsulfonyl-acrylamides with arylsulfonohydrazides: synthesis of sulfonated oxindoles. Org. Biomol. Chem. 12, 6349–6353 (2014).
Shi, L., Wang, H., Yang, H. & Fu, H. Iron-catalyzed arylsulfonylation of activated alkenes. Synlett 26, 688–694 (2015).
Wang, H., Sun, S. & Cheng, J. Copper-catalyzed arylsulfonylation and cyclizative carbonation of N-(arylsulfonyl)acrylamides involving desulfonative arrangement toward sulfonated oxindoles. Org. Lett. 19, 5844–5847 (2017).
Kong, W., Casimiro, M., Fuentes, N., Merino, E. & Nevado, C. Metal-free aryltrifluoromethylation of activated alkenes. Angew. Chem. Int. Ed. 52, 13086–13090 (2013).
Hervieu, C. et al. Chiral arylsulfinylamides: all-in-one reagents for visible light-mediated asymmetric alkene aminoarylations. Preprint at ChemRxiv https://doi.org/10.26434/chemrxiv-2022-jvxj1 (2022).
Sephton, T., Large, J. M., Butterworth, S. & Greaney, M. F. Diarylamine synthesis via desulfinylative Smiles rearrangement. Org. Lett. 24, 1132–1135 (2022).
Yozo, M., Yuji, N. & Masayoshi, K. An electron spin resonance spectroscopic investigation of N-sulfinylaminyls. Chem. Lett. 7, 521–524 (1978).
Yozo, M. & Yuji, N. Electron spin resonance spectra of sulfinamidyl radicals and a comparison of hyperfine splitting constants with sulfenamidyl and sulfonamidyl radicals. Bull. Chem. Soc. Jpn 63, 1154–1159 (1990).
Baban, J. A. & Roberts, B. P. An electron spin resonance study of dialkylaminothiyl (R2NS·), dialkylaminosulphinyl (R2NSO), and alkyl(sulphinyl)aminyl [RNS(O)X] radicals. Radical addition to N-sulphinylamines. J. Chem. Soc. Perkin Trans. 2, 678–683 (1978).
Xue, F. et al. A desulfurative strategy for the generation of alkyl radicals enabled by visible-light photoredox catalysis. Angew. Chem. Int. Ed. 57, 6667–6671 (2018).
Di, J. et al. Regiospecific alkyl addition of (hetero)arene-fused thiophenes enabled by a visible-light-mediated photocatalytic desulfuration approach. Chem. Commun. 54, 4692–4695 (2018).
Litwinienko, G. & Ingold, K. U. Abnormal solvent effects on hydrogen atom abstraction. 2. Resolution of the curcumin antioxidant controversy. The role of sequential proton loss electron transfer. J. Org. Chem. 69, 5888–5896 (2004).
Nicewicz, D., Roth, H. & Romero, N. Experimental and calculated electrochemical potentials of common organic molecules for applications to single-electron redox chemistry. Synlett 27, 714–723 (2015).
Devery, J. J., Nguyen, J. D., Dai, C. & Stephenson, C. R. J. Light-mediated reductive debromination of unactivated alkyl and aryl bromides. ACS Catal. 6, 5962–5967 (2016).
De Vleeschouwer, F., Van Speybroeck, V., Waroquier, M., Geerlings, P. & De Proft, F. Electrophilicity and nucleophilicity index for radicals. Org. Lett. 9, 2721–2724 (2007).
Herron, J. T. & Huie, R. E. Rate constants at 298 K for the reactions SO + SO + M → (SO)2 + M and SO + (SO)2 → SO2 + S2O. Chem. Phys. Lett. 76, 322–324 (1980).
So, C. M., Kume, S. & Hayashi, T. Rhodium-catalyzed asymmetric hydroarylation of 3-pyrrolines giving 3-arylpyrrolidines: protonation as a key step. J. Am. Chem. Soc. 135, 10990–10993 (2013).
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R35GM144286. We thank the University of Michigan for additional funding and J. W. Kampf for collecting and analysing X-ray crystallography data.
Author information
Authors and Affiliations
Contributions
E.A.N. and C.R.J.S. conceived the project, and E.A.N. executed the initial reaction discovery. E.A.N., C.H.N. and R.M.W. conducted the experiments and analysis. E.A.N. wrote the paper with input from C.R.J.S., C.H.N. and R.M.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Robert Stockman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–200, experimental procedures and details, characterization data, NMR spectra, chiral high-performance liquid chromatography and supercritical fluid chromatography traces and X-ray crystallographic data.
Supplementary Data 1
Crystallographic data for compound 2u. CCDC reference 2150974.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noten, E.A., Ng, C.H., Wolesensky, R.M. et al. A general alkene aminoarylation enabled by N-centred radical reactivity of sulfinamides. Nat. Chem. 16, 599–606 (2024). https://doi.org/10.1038/s41557-023-01404-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01404-w