Abstract
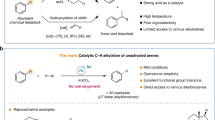
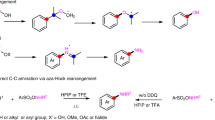
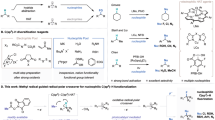
The photoactivation of electron donor–acceptor complexes has emerged as a sustainable, selective and versatile strategy for the generation of radical species. However, when it comes to aryl radical formation, this strategy remains hamstrung by the electronic properties of the aromatic radical precursors, and electron-deficient aryl halide acceptors are required. This has prevented the implementation of a general synthetic platform for aryl radical formation. Our study introduces triarylsulfonium salts as acceptors in photoactive electron donor–acceptor complexes, used in combination with catalytic amounts of newly designed amine donors. The sulfonium salt label renders inconsequential the electronic features of the aryl radical precursor and, more importantly, it is installed regioselectively in native aromatic compounds by C–H sulfenylation. Using this general, site-selective aromatic C–H functionalization approach, we developed metal-free protocols for the alkylation and cyanation of arenes, and showcased their application in both the synthesis and the late-stage modification of pharmaceuticals and agrochemicals.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Materials and methods, experimental procedures, useful information, mechanistic studies, 1H NMR spectra, 13C NMR spectra and mass spectrometry data are available in the Supplementary Information. Crystallographic data for compounds 2, 31, 33, 44, 64 and 69 have been deposited with the Cambridge Crystallographic Data Centre, with deposition numbers CCDC 2120242, 2120244, 2120243, 2122516, 2122517 and 2120245, respectively. Correspondence and requests for materials/raw data should be addressed to the corresponding author).
References
Sumida, Y. & Ohmiya, H. Direct excitation strategy for radical generation in organic synthesis. Chem. Soc. Rev. 50, 6320–6332 (2021).
Silvi, M. & Melchiorre, P. Enhancing the potential of enantioselective organocatalysis with light. Nature 554, 41–49 (2018).
Genzink, M. J., Kidd, J. B., Swords, W. B. & Yoon, T. P. Chiral photocatalyst structures in asymmetric photochemical synthesis. Chem. Rev. 122, 1654–1716 (2022).
Sandoval, B. A. et al. Photoenzymatic reductions enabled by direct excitation of flavin-dependent ‘ene’-reductases. J. Am. Chem. Soc. 143, 1735–1739 (2021).
Romero, N. A. & Nicewicz, D. A. Organic photoredox catalysis. Chem. Rev. 116, 10075–10166 (2016).
Shaw, M. H., Twilton, J. & MacMillan, D. W. C. Photoredox catalysis in organic chemistry. J. Org. Chem. 81, 6898–6926 (2016).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Crisenza, G. E. M., Mazzarella, D. & Melchiorre, P. Synthetic methods driven by the photoactivity of electron donor−acceptor complexes. J. Am. Chem. Soc. 142, 5461–5476 (2020).
Wu, J., Grant, P. S., Li, X., Noble, A. & Aggarwal, V. K. Catalyst free deaminative functionalizations of primary amines by photoinduced single-electron transfer. Angew. Chem. Int. Ed. 58, 5697–5701 (2019).
Fu, M.-C., Shang, R., Zhao, B., Wang, B. & Fu, Y. Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide. Science 363, 1429–1434 (2019).
de Pedro Beato, E., Spinnato, D., Zhou, W. & Melchiorre, P. A general organocatalytic system for electron donor−acceptor complex photoactivation and its use in radical processes. J. Am. Chem. Soc. 143, 12304–12314 (2021).
Davies, J., Booth, S. G., Essafi, S., Dryfe, R. A. W. & Leonori, D. Visible-light-mediated generation of nitrogen-centered radicals: metal-free hydroimination and iminohydroxylation cyclization reactions. Angew. Chem. Int. Ed. 54, 14017–14021 (2015).
Tobisu, M., Furukawa, T. & Chatani, N. Visible light-mediated direct arylation of arenes and heteroarenes using diaryliodonium salts in the presence and absence of a photocatalyst. Chem. Lett. 42, 1203–1205 (2013).
Liu, B., Lim, C.-H. & Miyake, G. M. Visible-light-promoted C-S cross-coupling via intermolecular charge transfer. J. Am. Chem. Soc. 139, 13616–13619 (2017).
Berger, F. et al. Site-selective and versatile aromatic C−H functionalization by thianthrenation. Nature 567, 223–228 (2019).
Aukland, M. H., Šiaučiulis, M., West, A., Perry, G. J. P. & Procter, D. J. Metal-free photoredox-catalyzed formal C-H/C-H coupling of arenes enabled by interrupted Pummerer activation. Nat. Catal. 3, 163–169 (2020).
Péter, Á., Perry, G. J. P. & Procter, D. J. Radical C–C bond formation using sulfonium salts and light. Adv. Synth. Catal. 362, 2135–2142 (2020).
Juliá, F. et al. High site selectivity in electrophilic aromatic substitutions: mechanism of C–H thianthrenation. J. Am. Chem. Soc. 143, 16041–16054 (2021).
Pulis, A. P. & Procter, D. J. C–H coupling reactions directed by sulfoxides: teaching an old functional group new tricks. Angew. Chem. Int. Ed. 55, 9842–9860 (2016).
Trudel, V., Tien, C.-H., Trofimove, A. & Yudin, A. K. Interrupted reactions in chemical synthesis. Nat. Rev. Chem. 5, 604–623 (2021).
Spell, M. L. et al. A visible-light-promoted O-glycosylation with a thioglycoside donor. Angew. Chem. Int. Ed. 55, 6515–6519 (2016).
Chen, C., Wang, Z.-J., Lu, H., Zhao, Y. & Shi, Z. Generation of non-stabilized alkyl radicals from thianthrenium salts for C-B and C-C bond formation. Nat. Commun. 12, 4526 (2021).
Hamann, B. C. & Hartwig, J. F. Palladium-catalyzed direct α-arylation of ketones. Rate acceleration by sterically hindered chelating ligands and reductive elimination from a transition metal enolate complex. J. Am. Chem. Soc. 119, 12382–12383 (1997).
Escudero-Casao, M., Licini, G. & Orlandi, M. Enantioselective α-arylation of ketones via a novel Cu(I)–bis(phosphine) dioxide catalytic system. J. Am. Chem. Soc. 143, 3289–3294 (2021).
Zhao, D., Xu, P. & Ritter, T. Palladium-catalyzed late-stage direct arene cyanation. Chem 5, 97–107 (2019).
McManus, J. B. & Nicewicz, D. A. Direct C−H cyanation of arenes via organic photoredox catalysis. J. Am. Chem. Soc. 139, 2880–2883 (2017).
McCarthy, B. G. et al. Structure−property relationships for tailoring phenoxazines as reducing photoredox catalysts. J. Am. Chem. Soc. 140, 5088–5101 (2018).
Sreenath, K., Veettil Suneesh, C., Gopidas, K. R. & Flowers, R. A. II Generation of triarylamine radical cations through reaction of triarylamines with Cu(II) in acetonitrile. A kinetic investigation of the electron-transfer reaction. J. Phys. Chem. A 113, 6477–6483 (2009).
Lei, J., Huang, J. & Zhu, Q. Recent progress in imidoyl radical-involved reactions. Org. Biomol. Chem. 14, 2593–2602 (2016).
Stork, G. & Sher, P. M. Regiospecific trapping of radicals from cyclization reactions. Cyclic nitriles via isocyanide trapping. J. Am. Chem. Soc. 105, 6765–6766 (1983).
Leardini, R., Nanni, D. & Zanardi, G. Radical addition to isonitriles: a route to polyfunctionalized alkenes through a novel three-component radical cascade reaction. J. Org. Chem. 65, 2763–2772 (2000).
Pimparkar, S. et al. C–CN bond formation: an overview of diverse strategies. Chem. Commun. 57, 2210–2232 (2021).
Guillemard, L., Kaplaneris, N., Ackermann, L. & Johansson, M. J. Late-stage C–H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 5, 522–545 (2021).
Berger, F. & Ritter, T. Site-selective late-stage C–H functionalization via thianthrenium salts. Synlett 33, 339–345 (2022).
Zhou, G., Liu, X. A process for preparing febuxostat. CN patent 102964313A (2013).
Buzzetti, L., Crisenza, G. E. M. & Melchiorre, P. Mechanistic studies in photocatalysis. Angew. Chem. Int. Ed. 58, 3730–3747 (2019).
Buglioni, L., Mastandrea, M. M., Frontera, A. & Pericàs, M. A. Anion–π interactions in light-induced reactions: role in the amidation of (hetero)aromatic systems with activated N-aryloxyamides. Chem. Eur. J. 25, 11785–11790 (2019).
Acknowledgements
We thank EPSRC (PhD Studentship to L.v.D. and PDRA funding to A.D. (EP/T013419/1)), the Leverhulme Trust (PDRA funding to J.A.R.-A. (RPG-2016-360)) and the University of Manchester (Lectureship to G.E.M.C.) for their generous support. Additionally, we thank the Natrajan group for their assistance with the photophysical studies, and the Leonori group for insightful discussion.
Author information
Authors and Affiliations
Contributions
J.A.R.-A., G.E.M.C. and D.J.P. conceived the project. L.v.D. and A.D. designed and performed the experimental work, with contributions from J.A.R.-A., E.G. and G.E.M.C. All the authors contributed to the analysis and interpretation of data. G.E.M.C. and D.J.P. wrote the manuscript with input from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5, Schemes 1–3, discussion and Tables 1–17.
Supplementary Data 1
Crystallographic data for compound 2; CCDC reference 2120242.
Supplementary Data 2
Crystallographic data for compound 31; CCDC reference 2120244.
Supplementary Data 3
Crystallographic data for compound 33; CCDC reference 2120243.
Supplementary Data 4
Crystallographic data for compound 44; CCDC reference 2122516.
Supplementary Data 5
Crystallographic data for compound 69; CCDC reference 2120245.
Supplementary Data 6
Crystallographic data for compound 64; CCDC reference 2122517.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dewanji, A., van Dalsen, L., Rossi-Ashton, J.A. et al. A general arene C–H functionalization strategy via electron donor–acceptor complex photoactivation. Nat. Chem. 15, 43–52 (2023). https://doi.org/10.1038/s41557-022-01092-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01092-y
This article is cited by
-
Radical arenes
Nature Chemistry (2023)
-
Altogether changed, and yet the same
Nature Chemistry (2023)
-
Metal-organic framework boosts heterogeneous electron donor–acceptor catalysis
Nature Communications (2023)