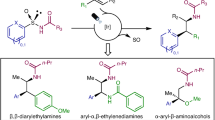
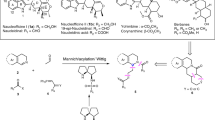
Abstract
Atropisomerism is a type of conformational chirality that plays a critical role in various fields of chemistry, including synthetic, medicinal and material chemistry, and its impact has been widely recognized. Although chiral atropisomerism in rotationally restricted aryl–aryl bonds has garnered substantial interest and led to important discoveries in chiral catalysts and drug development, the exploration of non-aryl atropisomers has fallen behind. Here we reveal a previously unexplored form of non-aryl atropisomerism by linking a sterically congested olefin to a sulfoxonium ylide. A streamlined synthetic approach to these novel molecules was developed through the hydrofunctionalization of alkynyl sulfoxonium ylides. Notably, an enantioselective organocatalytic strategy was developed to prepare these non-aryl atropisomers in high optical purity. This form of atropisomerism offers new routes for investigating the functional properties of axially chiral molecules.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2199007 (1), 2199008 (44), 2255173 (73), 2255174 (86) and 2259612 (88). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Experimental procedures, characterization of new compounds and all other data supporting the findings are available in the Supplementary Information.
References
Miyashita, A. et al. Synthesis of 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl (BINAP), an atropisomeric chiral bis(triaryl)phosphine, and its use in the rhodium(I)-catalyzed asymmetric hydrogenation of α-(acylamino)acrylic acids. J. Am. Chem. Soc. 102, 7932–7934 (1980).
Akiyama, T. & Mori, K. Stronger Brønsted acids: recent progress. Chem. Rev. 115, 9277–9306 (2015).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Cheng, J. K., Xiang, S.-H., Li, S., Ye, L. & Tan, B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 121, 4805–4902 (2021).
Wencel-Delord, J., Panossian, A., Leroux, F. R. & Colobert, F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 44, 3418–3430 (2015).
Cheng, D.-J. & Shao, Y.-D. Advances in the catalytic asymmetric synthesis of atropisomeric hexatomic N-heterobiaryls. Adv. Synth. Catal. 362, 3081–3099 (2020).
Wang, Y.-B. & Tan, B. Construction of axially chiral compounds via asymmetric organocatalysis. Acc. Chem. Res. 51, 534–547 (2018).
Kumarasamy, E., Raghunathan, R., Sibi, M. P. & Sivaguru, J. Nonbiaryl and heterobiaryl atropisomers: molecular templates with promise for atropselective chemical transformations. Chem. Rev. 115, 11239–11300 (2015).
Wu, S., Xiang, S.-H., Cheng, J. K. & Tan, B. Axially chiral alkenes: atroposelective synthesis and applications. Tetrahedron Chem 1, 100009 (2022).
Mei, G.-J., Koay, W. L., Guan, C.-Y. & Lu, Y. X. Atropisomers beyond the C–C axial chirality: advances in catalytic asymmetric synthesis. Chem 8, 1855–1893 (2022).
Faseke, V. C. & Sparr, C. Stereoselective arene-forming aldol condensation: synthesis of axially chiral aromatic amides. Angew. Chem. Int. Ed. 55, 7261–7264 (2016).
Fugard, A. J. et al. Hydrogen-bond-enabled dynamic kinetic resolution of axially chiral amides mediated by a chiral counterion. Angew. Chem. Int. Ed. 58, 2795–2798 (2019).
Barrett, K. T., Metrano, A. J., Rablen, P. R. & Miller, S. J. Spontaneous transfer of chirality in an atropisomerically enriched two-axis system. Nature 509, 71–75 (2014).
Frey, J. et al. Enantioselective synthesis of N–C axially chiral compounds by Cu-catalyzed atroposelective aryl amination. Angew. Chem. Int. Ed. 59, 8844–8848 (2020).
Bai, H.-Y. et al. Highly atroposelective synthesis of nonbiaryl naphthalene-1,2-diamine N–C atropisomers through direct enantioselective C–H amination. Nat. Commun. 10, 3063 (2019).
Li, S.-L. et al. Atroposelective catalytic asymmetric allylic alkylation reaction for axially chiral anilides with achiral Morita–Baylis–Hillman carbonates. J. Am. Chem. Soc. 140, 12836–12843 (2018).
Li, H. et al. Enantioselective synthesis of C–N axially chiral N-aryloxindoles by asymmetric rhodium-catalyzed dual C–H activation. Angew. Chem. Int. Ed. 58, 6732–6736 (2019).
Zhu, S. et al. Organocatalytic atroposelective construction of axially chiral arylquinones. Nat. Commun. 10, 4268 (2019).
Chen, Y.-H. et al. Organocatalytic enantioselective synthesis of atropisomeric aryl-p-quinones: platform molecules for diversity-oriented synthesis of biaryldiols. Angew. Chem. Int. Ed. 59, 11374–11378 (2020).
Feng, J., Li, B., He, Y. & Gu, Z. Enantioselective synthesis of atropisomeric vinyl arene compounds by palladium catalysis: a carbene strategy. Angew. Chem. Int. Ed. 55, 2186–2190 (2016).
Pan, C., Zhu, Z., Zhang, M. & Gu, Z. Palladium-catalyzed enantioselective synthesis of 2-aryl cyclohex-2-enone atropisomers: platform molecules for the divergent synthesis of axially chiral biaryl compounds. Angew. Chem. Int. Ed. 56, 4777–4781 (2017).
Liang, Y. et al. Enantioselective construction of axially chiral amino sulfide vinyl arenes by chiral sulfide-catalyzed electrophilic carbothiolation of alkynes. Angew. Chem. Int. Ed. 59, 4959–4964 (2020).
Fang, Z.-J. et al. Asymmetric synthesis of axially chiral isoquinolones: nickel-catalyzed denitrogenative transannulation. Angew. Chem. Int. Ed. 54, 9528–9532 (2015).
Zheng, S.-C. et al. Organocatalytic atroposelective synthesis of axially chiral styrenes. Nat. Commun. 8, 15238 (2017).
Zhang, N. et al. Organocatalytic atropo- and E/Z-selective Michael addition reaction of ynones with α-amido sulfones as sulfone-type nucleophile. Org. Chem. Front. 6, 451–455 (2019).
Yan, J.-L. et al. Carbene-catalyzed atroposelective synthesis of axially chiral styrenes. Nat. Commun. 13, 84 (2022).
Boer, F. P., Doorakian, G. A., Freedman, H. H. & McKinley, S. V. Study of the rotational process in sterically hindered dienes. J. Am. Chem. Soc. 92, 1225–1233 (1970).
Boer, F. P., Flynn, J. J., Freedman, H. H., McKinley, S. V. & Sandel, V. R. Hindered diene rotation by virtue of intramolecular tin–bromine coordination. J. Am. Chem. Soc. 89, 5068–5069 (1967).
Rösner, M. & Köbrich, G. Partial enantiomerization of an atropisomeric butadiene. Angew. Chem. Int. Ed. 13, 741–742 (1974).
Pasto, D. J. & Borchardt, J. K. Cycloaddition reactions of cyclopropane-containing systems. VII. Chiral 1,2-bisalkylidenecyclopentanes. Direct formation via cycloaddition reactions of chiral substituted alkenylidenecyclopropanes. J. Am. Chem. Soc. 96, 6220–6221 (1974).
Warren, S., Chow, A., Fraenkel, G. & RajanBabu, T. V. Axial chirality in 1,4-disubstituted (ZZ)-1,3-dienes. Surprisingly low energies of activation for the enantiomerization in synthetically useful fluxional molecules. J. Am. Chem. Soc. 125, 15402–15410 (2003).
Zhang, Z.-X., Zhai, T.-Y. & Ye, L.-W. Synthesis of axially chiral compounds through catalytic asymmetric reactions of alkynes. Chem. Catal. 1, 1378–1412 (2021).
Zhang, L. et al. Design and atroposelective construction of IAN analogues by organocatalytic asymmetric heteroannulation of alkynes. Angew. Chem. Int. Ed. 59, 23077–23082 (2020).
Wang, Y.-B. et al. Rational design, enantioselective synthesis and catalytic applications of axially chiral EBINOLs. Nat. Catal. 2, 504–513 (2019).
Huang, A. et al. Asymmetric one-pot construction of three stereogenic elements: chiral carbon center, stereoisomeric alkenes, and chirality of axial styrenes. Org. Lett. 21, 95–99 (2019).
Jia, S. et al. Organocatalytic cascade reactions for multi-functionalized chiral cyclic ethers through vinylidene ortho-quinone methides. Chem. Commun. 57, 11334–11337 (2021).
Liu, Y. et al. Organocatalytic atroposelective intramolecular [4 + 2] cycloaddition: synthesis of axially chiral heterobiaryls. Angew. Chem. Int. Ed. 57, 6491–6495 (2018).
Jia, S., Li, S., Liu, Y., Qin, W. & Yan, H. Enantioselective control of both helical and axial stereogenic elements though an organocatalytic approach. Angew. Chem. Int. Ed. 58, 18496–18501 (2019).
Peng, L. et al. Organocatalytic asymmetric annulation of ortho-alkynylanilines: synthesis of axially chiral naphthyl-C2-indoles. Angew. Chem. Int. Ed. 58, 17199–17204 (2019).
Jia, S. et al. Organocatalytic enantioselective construction of axially chiral sulfone-containing styrenes. J. Am. Chem. Soc. 140, 7056–7060 (2018).
Qin, W., Liu, Y. & Yan, H. Enantioselective synthesis of atropisomers via vinylidene ortho-quinone methides (VQMs). Acc. Chem. Res. 55, 2780–2795 (2022).
Huang, S. et al. Metal-free difunctionalization of alkynes to access tetrasubstituted olefins through spontaneous selenosulfonylation of vinylidene ortho-quinone methide (VQM). Org. Biomol. Chem. 17, 1121–1129 (2019).
Tan, Y. et al. Enantioselective construction of vicinal diaxial styrenes and multiaxis system via organocatalysis. J. Am. Chem. Soc. 140, 16893–16898 (2018).
Furusawa, M. et al. Base-catalyzed Schmittel cycloisomerization of o-phenylenediyne-linked bis(arenol)s to indeno[1,2-c]chromenes. Tetrahedron Lett. 54, 7107–7110 (2013).
Li, Q.-Z. et al. Organocatalytic enantioselective construction of heterocycle-substituted styrenes with chiral atropisomerism. Org. Lett. 22, 2448–2453 (2020).
Xu, M.-M. et al. Enantioselective synthesis of axially chiral biaryls by Diels–Alder/retro-Diels–Alder reaction of 2-pyrones with alkynes. J. Am. Chem. Soc. 143, 8993–9001 (2021).
Zhang, X. et al. Asymmetric azide–alkyne cycloaddition with Ir(I)/squaramide cooperative catalysis: atroposelective synthesis of axially chiral aryltriazoles. J. Am. Chem. Soc. 144, 6200–6207 (2022).
Li, L. et al. Synthesis of I(III)/S(VI) reagents and their reactivity in photochemical cycloaddition reactions with unsaturated bonds. Nat. Commun. 13, 6588 (2022).
Gallo, R. D. C., Ahmad, A., Metzker, G. & Burtoloso, A. C. B. α,α-Alkylation–halogenation and dihalogenation of sulfoxonium ylides. A direct preparation of geminal difunctionalized ketones. Chem. Eur. J. 23, 16980–16984 (2017).
Mi, R. et al. Rhodium-catalyzed atroposelective access to axially chiral olefins via C–H bond activation and directing group migration. Angew. Chem. Int. Ed. 61, e202111860 (2022).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21825101), Hong Kong RGC (16302122, 16300320 and 16309021) and the Shenzhen Science and Technology Innovation Commission (SGDX2019081623241924). We thank Z. Lin for insightful discussions.
Author information
Authors and Affiliations
Contributions
F.W. and Y.H. designed the project. Y.H. directed the project. F.W. conducted the experiments. R.Z. conducted the transformation experiments. Y.Z. performed computation of the rotational barriers and the mechanism study. All authors contributed to designing, performing and analysing the experiments, as well as to the writing of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Dao-Juan Cheng, Michele Mancinelli and Takanori Shibata for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–9, Figs. 1–12, Tables 1–44 and refs. 1–18.
Supplementary Data 1
Crystallographic data for compound 1; CCDC reference 2199007.
Supplementary Data 2
Crystallographic data for compound 44; CCDC reference 2199008.
Supplementary Data 3
Crystallographic data for compound 73; CCDC reference 2255173.
Supplementary Data 4
Crystallographic data for compound 86; CCDC reference 2255174.
Supplementary Data 5
Crystallographic data for compound 88; CCDC reference 2259612.
Supplementary Data 6
Computational data for DFT calculations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, F., Zhang, Y., Zhu, R. et al. Discovery and synthesis of atropisomerically chiral acyl-substituted stable vinyl sulfoxonium ylides. Nat. Chem. 16, 132–139 (2024). https://doi.org/10.1038/s41557-023-01358-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01358-z