Abstract
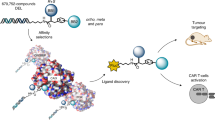
DNA-encoded chemical libraries (DELs) consist of large chemical compound collections individually linked to DNA barcodes, facilitating pooled construction and screening. However, screening campaigns often fail if the molecular arrangement of the building blocks is not conducive to an efficient interaction with a protein target. Here we postulated that the use of rigid, compact and stereo-defined central scaffolds for DEL synthesis may facilitate the discovery of very specific ligands capable of discriminating between closely related protein targets. We synthesized a DEL comprising 3,735,936 members, featuring the four stereoisomers of 4-aminopyrrolidine-2-carboxylic acid as central scaffolds. The library was screened in comparative selections against pharmaceutically relevant targets and their closely related protein isoforms. Hit validation results revealed a strong impact of stereochemistry, with large affinity differences between stereoisomers. We identified potent isozyme-selective ligands against multiple protein targets. Some of these hits, specific to tumour-associated antigens, demonstrated tumour-selective targeting in vitro and in vivo. Collectively, constructing DELs with stereo-defined elements contributed to high library productivity and ligand selectivity.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article and Supplementary Information. Next-generation sequencing data of all selection duplicates are provided as separate text files. Source Data Figs. 1–6 and Supplementary Figs. 14, 19, 20, 43–46 and 53–63 are provided with the paper. The Fastq file, containing raw high throughput Illumina sequence counts, is not provided in this article and Supplementary Information, but can be made available to readers upon justified request addressed to the corresponding authors. Source data are provided with this paper.
Code availability
Software for the evaluation of HTDS has previously been reported5.
References
Hughes, J. P., Rees, S., Kalindjian, S. B. & Philpott, K. L. Principles of early drug discovery. Br. J. Pharmacol. 162, 1239–1249 (2011).
Macarron, R. et al. Impact of high-throughput screening in biomedical research. Nat. Rev. Drug Discov. 10, 188–195 (2011).
Goodnow, R. A., Dumelin, C. E. & Keefe, A. D. DNA-encoded chemistry: enabling the deeper sampling of chemical space. Nat. Rev. Drug Discov. 16, 131–147 (2017).
Neri, D. & Lerner, R. A. DNA-encoded chemical libraries: a selection system based on endowing organic compounds with amplifiable information. Annu. Rev. Biochem. 87, 479–502 (2018).
Decurtins, W. et al. Automated screening for small organic ligands using DNA-encoded chemical libraries. Nat. Protoc. 11, 764–780 (2016).
Mannocci, L. et al. High-throughput sequencing allows the identification of binding molecules isolated from DNA-encoded chemical libraries. Proc. Natl Acad. Sci. USA 105, 17670–17675 (2008).
Buller, F. et al. Design and synthesis of a novel DNA-encoded chemical library using Diels–Alder cycloadditions. Bioorg. Med. Chem. Lett. 18, 5926–5931 (2008).
Clark, M. A. et al. Design, synthesis and selection of DNA-encoded small-molecule libraries. Nat. Chem. Biol. 5, 647–654 (2009).
Favalli, N. et al. Stereo- and regiodefined DNA-encoded chemical libraries enable efficient tumour-targeting applications. Nat. Chem. 13, 540–548 (2021).
Li, Y. et al. Versatile protein recognition by the encoded display of multiple chemical elements on a constant macrocyclic scaffold. Nat. Chem. 10, 441–448 (2018).
Gartner, Z. J. et al. DNA-templated organic synthesis and selection of a library of macrocycles. Science 305, 1601–1605 (2004).
Li, Y., Zhao, P., Zhang, M., Zhao, X. & Li, X. Multistep DNA-templated synthesis using a universal template. J. Am. Chem. Soc. 135, 17727–17730 (2013).
Petersen, L. K. et al. Novel p38α MAP kinase inhibitors identified from yoctoReactor DNA-encoded small molecule library. MedChemComm 7, 1332–1339 (2016).
Melkko, S., Scheuermann, J., Dumelin, C. E. & Neri, D. Encoded self-assembling chemical libraries. Nat. Biotechnol. 22, 568–574 (2004).
Wichert, M. et al. Dual-display of small molecules enables the discovery of ligand pairs and facilitates affinity maturation. Nat. Chem. 7, 241–249 (2015).
Reddavide, F. V., Lin, W., Lehnert, S. & Zhang, Y. DNA-encoded dynamic combinatorial chemical libraries. Angew. Chem. Int. Ed. 54, 7924–7928 (2015).
Li, G. et al. Design, preparation, and selection of DNA-encoded dynamic libraries. Chem. Sci. 6, 7097–7104 (2015).
Litovchick, A. et al. Encoded library synthesis using chemical ligation and the discovery of sEH inhibitors from a 334-million member library. Sci Rep. 5, 10916 (2015).
Bassi, G. et al. A single-stranded DNA-encoded chemical library based on a stereoisomeric scaffold enables ligand discovery by modular assembly of building blocks. Adv. Sci. 7, 2001970 (2020).
Sannino, A. et al. Quantitative assessment of affinity selection performance using DNA-encoded chemical libraries. ChemBioChem 20, 955–962 (2018).
Santos, R. et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 16, 19–34 (2017).
Huggins, D. J., Sherman, W. & Tidor, B. Rational approaches to improving selectivity in drug design. J. Med. Chem. 55, 1424–1444 (2012).
Tafreshi, N. K., Lloyd, M. C., Bui, M. M., Gillies, R. J. & Morse, D. L. Carbonic anhydrase IX as an imaging and therapeutic target for tumors and metastases. Subcell. Biochem. 75, 221–254 (2014).
Aggarwal, M., Boone, C. D., Kondeti, B. & McKenna, R. Structural annotation of human carbonic anhydrases. J. Enzyme Inhib. Med. Chem. 28, 267–277 (2013).
Supuran, C. T. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 7, 168–181 (2008).
Cazzamalli, S., Dal Corso, A. & Neri, D. Acetazolamide serves as selective delivery vehicle for dipeptide-linked drugs to renal cell carcinoma. Mol. Cancer Ther. 15, 2926–2935 (2016).
Kulterer, O. C. et al. A microdosing study with 99mTc-PHC-102 for the SPECT/CT imaging of primary and metastatic lesions in renal cell carcinoma patients. J. Nucl. Med. 62, 360 (2021).
Zhong, L. et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 6, 201 (2021).
Sartor, O. et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 385, 1091–1103 (2021).
Heynickx, N., Herrmann, K., Vermeulen, K., Baatout, S. & Aerts, A. The salivary glands as a dose limiting organ of PSMA-targeted radionuclide therapy: a review of the lessons learnt so far. Nucl. Med. Biol. 98-99, 30–39 (2021).
Lucaroni, L. et al. Cross-reactivity to glutamate carboxypeptidase III causes undesired salivary gland and kidney uptake of PSMA-targeted small-molecule radionuclide therapeutics. Eur. J. Nucl. Med. Mol. Imaging 50, 957–961 (2023).
Bassi, G. et al. Specific inhibitor of placental alkaline phosphatase isolated from a DNA-encoded chemical library targets tumor of the female reproductive tract. J. Nucl. Med. 64, 15799–15809 (2021).
Gerry, C. J., Wawer, M. J., Clemons, P. A. & Schreiber, S. L. DNA barcoding a complete matrix of stereoisomeric small molecules. J. Am. Chem. Soc. 141, 10225–10235 (2019).
Benhamou, R. I. et al. DNA-encoded library versus RNA-encoded library selection enables design of an oncogenic noncoding RNA inhibitor. Proc. Natl Acad. Sci. USA 119, e2114971119 (2022).
Favalli, N. et al. A DNA-encoded library of chemical compounds based on common scaffolding structures reveals the impact of ligand geometry on protein recognition. ChemMedChem 13, 1303–1307 (2018).
Levy, M. & Ellington, A. D. Directed evolution of streptavidin variants using in vitro compartmentalization. Chem. Biol. 15, 979–989 (2008).
Supuran, C. T., Briganti, F., Tilli, S., Chegwidden, W. R. & Scozzafava, A. Carbonic anhydrase inhibitors: sulfonamides as antitumor agents? Bioorg. Med. Chem. 9, 703–714 (2001).
Vintonyak, V. V., Antonchick, A. P., Rauh, D. & Waldmann, H. The therapeutic potential of phosphatase inhibitors. Curr. Opin. Chem. Biol. 13, 272–283 (2009).
Bigatti, M. et al. Impact of a central scaffold on the binding affinity of fragment pairs isolated from DNA-encoded self-assembling chemical libraries. ChemMedChem 12, 1748–1752 (2017).
Hoylaerts, M. F., Ding, L., Narisawa, S., Van kerckhoven, S. & Millán, J. L. Mammalian alkaline phosphatase catalysis requires active site structure stabilization via the N-terminal amino acid microenvironment. Biochemistry 45, 9756–9766 (2006).
Kramer, V. et al. Biodistribution and dosimetry of a single dose of albumin-binding ligand [(177)Lu]Lu-PSMA-ALB-56 in patients with mCRPC. Eur. J. Nucl. Med. Mol. Imaging 48, 893–903 (2021).
Lucaroni, L. et al. Cross-reactivity to glutamate carboxypeptidase III causes undesired salivary gland and kidney uptake of PSMA-targeted small molecule radionuclide therapeutics, Eur. J. Nucl. Med. Mol. Imaging (2022), in revision.
Kelly, J. M. et al. Albumin-binding PSMA ligands: implications for expanding the therapeutic window. J. Nucl. Med. 60, 656 (2019).
Dumelin, C. E. et al. A portable albumin binder from a DNA-encoded chemical library. Angew. Chem. Int. Ed. 47, 3196–3201 (2008).
Tan, D. S., Foley, M. A., Shair, M. D. & Schreiber, S. L. Stereoselective synthesis of over two million compounds having structural features both reminiscent of natural products and compatible with miniaturized cell-based assays. J. Am. Chem. Soc. 120, 8565–8566 (1998).
Scott, K. A. et al. Stereochemical diversity as a source of discovery in chemical biology. Curr. Res. Chem. Biol. 2, 100028 (2022).
Ferraroni, M., Cornelio, B., Sapi, J., Supuran, C. T. & Scozzafava, A. Sulfonamide carbonic anhydrase inhibitors: zinc coordination and tail effects influence inhibitory efficacy and selectivity for different isoforms. Inorg. Chim. Acta 470, 128–132 (2018).
Cazzamalli, S., Corso, A. D. & Neri, D. Linker stability influences the anti-tumor activity of acetazolamide-drug conjugates for the therapy of renal cell carcinoma. J. Control. Release 246, 39–45 (2017).
Cazzamalli, S. et al. In vivo antitumor activity of a novel acetazolamide–cryptophycin conjugate for the treatment of renal cell carcinomas. ACS Omega 3, 14726–14731 (2018).
Pellegrino, C. et al. Impact of ligand size and conjugation chemistry on the performance of universal chimeric antigen receptor T-cells for tumor killing. Bioconjug. Chem. 31, 1775–1783 (2020).
Tölle, M., Reshetnik, A., Schuchardt, M., Höhne, M. & van der Giet, M. Arteriosclerosis and vascular calcification: causes, clinical assessment and therapy. Eur. J. Clin. Invest. 45, 976–985 (2015).
Opdebeeck, B. et al. Pharmacological TNAP inhibition efficiently inhibits arterial media calcification in a warfarin rat model but deserves careful consideration of potential physiological bone formation/mineralization impairment. Bone 137, 115392 (2020).
Safety profile. PLUVICTO https://www.hcp.novartis.com/products/pluvicto/psma-positive-mcrpc/safety-profile/ (2022).
Kopka, K., Benešová, M., Bařinka, C., Haberkorn, U. & Babich, J. Glu-ureido–based inhibitors of prostate-specific membrane antigen: lessons learned during the development of a novel class of low-molecular-weight theranostic radiotracers. J. Nucl. Med. 58, 17S–26S (2017).
Srinivasarao, M., Galliford, C. V. & Low, P. S. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat. Rev. Drug Discov. 14, 203–219 (2015).
Stefanelli, P. & Rezza, G. COVID-19 vaccination strategies and their adaptation to the emergence of SARS-CoV-2 variants. Vaccines 10, 905 (2022).
Taylor, P. C. et al. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 21, 382–393 (2021).
Xiang, R. et al. Recent advances in developing small-molecule inhibitors against SARS-CoV-2. Acta Pharm. Sin. B 12, 1591–1623 (2022).
Owen, D. R. et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 374, 1586–1593 (2021).
Minskaia, E. et al. Discovery of an RNA virus 3′→5′ exoribonuclease that is critically involved in coronavirus RNA synthesis. Proc. Natl Acad. Sci. USA 103, 5108–5113 (2006).
Ma, Y. et al. Structural basis and functional analysis of the SARS coronavirus nsp14–nsp10 complex. Proc. Natl Acad. Sci. USA 112, 9436–9441 (2015).
Ogando, N. S. et al. The enzymatic activity of the nsp14 exoribonuclease is critical for replication of MERS-CoV and SARS-CoV-2. J. Virol. 94, e01246–01220. (2020).
Devkota, K. et al. Probing the SAM binding site of SARS-CoV-2 nsp14 in vitro using SAM competitive inhibitors guides developing selective bi-substrate inhibitors. SLAS Discov. 26, 1200–1211 (2021).
Otava, T. et al. The structure-based design of SARS-CoV-2 nsp14 methyltransferase ligands yields nanomolar inhibitors. ACS Infect. Dis. 7, 2214–2220 (2021).
Hofman, G.-J. et al. Minimising conformational bias in fluoroprolines through vicinal difluorination. Chem. Commun. 54, 5118–5121 (2018).
Acknowledgements
The authors thank A. Galbiati and A. Zana for the support with the in vivo experiments. Furthermore, the authors thank C. Pellegrino for the help with flow cytometry measurements. The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the manuscript preparation (S. O., L. L., F. M., A. E., L. P., S. P., M. M., K. S., J. S., D. Y., M. J., N. B., D. B., R. K., S. C., D. N., N. F. and G. B.). S.O., G.B., N.F., S.C. and D.N. designed the experiments. S.O. synthesized the library with support from G.B., N.F. and F.M. S.O. and G.B. performed the selections. S.O., G.B., N.F. and J.S. analysed the HTS data. Hits were synthesized and validated by S.O. and G.B. with support from L.P., L.L., S.P., N.F. and A.E. A.E., L.L., S.O., M.M., L.P., G.B. and N.F. expressed and biotinylated the proteins with exception of Nsp14, which was provided by N.B., M.J., D.Y. and K.S. In vitro and in vivo experiments were performed by S.O., G.B. and S.C.
Corresponding authors
Ethics declarations
Competing interests
D.N. is co-founder, CEO, CSO and President of the Scientific Advisory Board of Philogen. S.O., L.L., A.E., F.M., S.P., M.M., L.P., S.C., N.F. and G.B. are employed by Philochem AG, the research and development unit of the Philogen. R.K. is member of the Board of Directors Chair and Scientific Advisory Board of NeoTX Therapeutics LTD. D.B. is CSO of the Discovery Division at NeoTX Therapeutics LTD. All other authors do not declare any conflict of interest.
Peer review
Peer review information
Nature Chemistry thanks Christian Heinis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Supplementary information
Supplementary Information
Supplementary Figs. 1–279, Supplementary Tables 1–7, results, experimental data, procedural details, synthesis and characterization data, NMR spectra, mass spectrometry spectra and source data files.
Supplementary Table 1
Source data file for supplementary figures.
Supplementary Data 1
Next-generation sequencing raw data of all selection duplicates are provided as separate text files.
Source data
Source Data Fig. 2
Numerical source data of FP measurements, SPR sensograms, affinity constant values and quantitative biodistributions.
Source Data Fig. 3
Numerical source data of FP measurements.
Source Data Fig. 4
Numerical source data of FP measurements and of the co-elution experiment.
Source Data Fig. 4
Unprocessed microscope image of immunofluorescence staining of human submandibular salivary glands with PSMA-617*.
Source Data Fig. 4
Unprocessed microscope image of immunofluorescence staining of human submandibular salivary glands with compound 45.
Source Data Fig. 5
Numerical source data of FP measurements.
Source Data Fig. 6
Numerical source data of FP measurements and affinity constant values.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oehler, S., Lucaroni, L., Migliorini, F. et al. A DNA-encoded chemical library based on chiral 4-amino-proline enables stereospecific isozyme-selective protein recognition. Nat. Chem. 15, 1431–1443 (2023). https://doi.org/10.1038/s41557-023-01257-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01257-3