Abstract
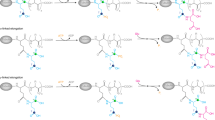
Microtubules, a critical component of the cytoskeleton, carry post-translational modifications (PTMs) that are important for the regulation of key cellular processes. Long-lived microtubules, in neurons particularly, exhibit both detyrosination of α-tubulin and polyglutamylation. Dysregulation of these PTMs can result in developmental defects and neurodegeneration. Owing to a lack of tools to study the regulation and function of these PTMs, the mechanisms that govern such PTM patterns are not well understood. Here we produce fully functional tubulin carrying precisely defined PTMs within its C-terminal tail. We ligate synthetic α-tubulin tails—which are site-specifically glutamylated—to recombinant human tubulin heterodimers by applying a sortase- and intein-mediated tandem transamidation strategy. Using microtubules reconstituted with these designer tubulins, we find that α-tubulin polyglutamylation promotes its detyrosination by enhancing the activity of the tubulin tyrosine carboxypeptidase vasohibin/small vasohibin-binding protein in a manner dependent on the length of polyglutamyl chains. We also find that modulating polyglutamylation levels in cells results in corresponding changes in detyrosination, corroborating the link between the detyrosination cycle to polyglutamylation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data associated with this study are presented in the article, extended data, source data or Supplementary Information. The data generated during the study are also available from the corresponding author on reasonable request. Final microtubule maps were submitted EMDB with codes EMD-15118, EMD-15119 and EMD-15120. Source Data are provided with this paper.
References
Goodson, H. V. & Jonasson, E. M. Microtubules and microtubule-associated proteins. Cold Spring Harb. Perspect. Biol. 10, a022608 (2018).
Janke, C. & Magiera, M. M. The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 21, 307–326 (2020).
Yu, I., Garnham, C. P. & Roll-Mecak, A. Writing and reading the tubulin code. J. Biol. Chem. 290, 17163–17172 (2015).
Gadadhar, S., Bodakuntla, S., Natarajan, K. & Janke, C. The tubulin code at a glance. J. Cell Sci. 130, 1347–1353 (2017).
Garnham, C. P. & Roll-Mecak, A. The chemical complexity of cellular microtubules: tubulin post-translational modification enzymes and their roles in tuning microtubule functions. Cytoskeleton 69, 442–463 (2012).
Li, F. et al. Cryo-EM structure of VASH1-SVBP bound to microtubules. eLife 9, e58157 (2020).
Wang, N. et al. Structural basis of tubulin detyrosination by the vasohibin-SVBP enzyme complex. Nat. Struct. Mol. Biol. 26, 571–582 (2019).
Li, F., Hu, Y., Qi, S., Luo, X. & Yu, H. Structural basis of tubulin detyrosination by vasohibins. Nat. Struct. Mol. Biol. 26, 583–591 (2019).
Aillaud, C. et al. Vasohibins/SVBP are tubulin carboxypeptidases (TCPs) that regulate neuron differentiation. Science 358, 1448–1453 (2017).
Landskron, L. et al. Posttranslational modification of microtubules by the MATCAP detyrosinase. Science 376, eabn6020 (2022).
Akhmanova, A. & Maiato, H. Closing the tubulin detyrosination cycle. Science 358, 1381–1382 (2017).
Webster, D. R., Gundersen, G. G., Bulinski, J. C. & Borisy, G. G. Differential turnover of tyrosinated and detyrosinated microtubules. Proc Natl Acad. Sci. USA 84, 9040–9044 (1987).
Magiera, M. M. & Janke, C. Post-translational modifications of tubulin. Curr. Biol. 24, R351–R354 (2014).
Wloga, D. et al. Hyperglutamylation of tubulin can either stabilize or destabilize microtubules in the same cell. Eukaryot Cell 9, 184–193 (2010).
Schulze, E., Asai, D. J., Bulinski, J. C. & Kirschner, M. Posttranslational modification and microtubule stability. J. Cell Biol. 105, 2167–2177 (1987).
Bobinnec, Y. et al. Glutamylation of centriole and cytoplasmic tubulin in proliferating non-neuronal cells. Cell Motil. Cytoskeleton 39, 223–232 (1998).
Valenstein, M. L. & Roll-Mecak, A. Graded control of microtubule severing by tubulin glutamylation. Cell 164, 911–921 (2016).
Zheng, P. et al. ER proteins decipher the tubulin code to regulate organelle distribution. Nature 601, 132–138 (2022).
McKenney, R. J., Huynh, W., Vale, R. D. & Sirajuddin, M. Tyrosination of α-tubulin controls the initiation of processive dynein–dynactin motility. EMBO J. 35, 1175–1185 (2016).
Barisic, M. et al. Mitosis. Microtubule detyrosination guides chromosomes during mitosis. Science 348, 799–803 (2015).
Chakraborti, S., Natarajan, K., Curiel, J., Janke, C. & Liu, J. The emerging role of the tubulin code: from the tubulin molecule to neuronal function and disease. Cytoskeleton 73, 521–550 (2016).
Sirajuddin, M., Rice, L. M. & Vale, R. D. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat. Cell Biol. 16, 335–344 (2014).
Minoura, I. et al. Overexpression, purification, and functional analysis of recombinant human tubulin dimer. FEBS Lett. 587, 3450–3455 (2013).
Vila-Perelló, M. & Muir, T. Biological applications of protein splicing. Cell 143, 191–200 (2010).
Thompson, R. E., Stevens, A. J. & Muir, T. W. Protein engineering through tandem transamidation. Nat. Chem. 11, 737–743 (2019).
Stevens, A. J. et al. Design of a split intein with exceptional protein splicing activity. J. Am. Chem. Soc. 138, 2162–2165 (2016).
Stevens, A. J. et al. A promiscuous split intein with expanded protein engineering applications. Proc. Natl Acad. Sci. USA 114, 8538–8543 (2017).
Dorr, B. M., Ham, H. O., An, C., Chaikof, E. L. & Liu, D. R. Reprogramming the specificity of sortase enzymes. Proc. Natl Acad. Sci. USA 111, 13343–13348 (2014).
Zhang, Y., Park, K. Y., Suazo, K. F. & Distefano, M. D. Recent progress in enzymatic protein labelling techniques and their applications. Chem. Soc. Rev. 47, 9106–9136 (2018).
Ti, S. C. et al. Mutations in human tubulin proximal to the kinesin-binding site alter dynamic instability at microtubule plus- and minus-ends. Dev. Cell 37, 72–84 (2016).
Bieniossek, C., Richmond, T. J. & Berger, I. MultiBac: multigene baculovirus-based eukaryotic protein complex production. Curr Protoc Protein Sci https://doi.org/10.1002/0471140864.ps0520s51 (2008).
Schatz, P. J., Georges, G. E., Solomon, F. & Botstein, D. Insertions of up to 17 amino acids into a region of alpha-tubulin do not disrupt function in vivo. Mol. Cell. Biol. 7, 3799–3805 (1987).
Vemu, A. et al. Structure and dynamics of single-isoform recombinant neuronal human tubulin. J. Biol. Chem. 291, 12907–12915 (2016).
Dawson, P. E., Muir, T. W., Clark-Lewis, I. & Kent, S. B. H. Synthesis of proteins by native chemical ligation. Science 266, 776–779 (1994).
Sui, H. & Downing, K. H. Structural basis of interprotofilament interaction and lateral deformation of microtubules. Structure 18, 1022–1031 (2010).
Pamula, M. C., Ti, S. C. & Kapoor, T. M. The structured core of human beta tubulin confers isotype-specific polymerization properties. J. Cell Biol. 213, 425–433 (2016).
Vemu, A., Atherton, J., Spector, J. O., Moores, C. A. & Roll-Mecak, A. Tubulin isoform composition tunes microtubule dynamics. Mol Biol Cell 28, 3564–3572 (2017).
Panda, D., Miller, H. P., Banerjee, A., Luduena, R. F. & Wilson, L. Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proc. Natl Acad. Sci. USA 91, 11358–11362 (1994).
Venne, A. S., Kollipara, L. & Zahedi, R. P. The next level of complexity: crosstalk of posttranslational modifications. Proteomics 14, 513–524 (2014).
van Dijk, J. et al. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol Cell 26, 437–448 (2007).
Zhou, C., Yan, L., Zhang, W. H. & Liu, Z. Structural basis of tubulin detyrosination by VASH2/SVBP heterodimer. Nat. Commun. 10, 3212 (2019).
Edde, B. et al. Posttranslational glutamylation of alpha-tubulin. Science 247, 83–85 (1990).
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Angell, Y. L. & Burgess, K. Peptidomimetics via copper-catalyzed azide–alkyne cycloadditions. Chem. Soc. Rev. 36, 1674–1689 (2007).
Pedersen, D. S. & Abell, A. 1,2,3-Triazoles in peptidomimetic chemistry. Eur. J. Org. Chem. 2011, 2399–2411 (2011).
Nieuwenhuis, J. et al. Vasohibins encode tubulin detyrosinating activity. Science 358, 1453–1456 (2017).
Bodakuntla, S., Janke, C. & Magiera, M. M. Tubulin polyglutamylation, a regulator of microtubule functions, can cause neurodegeneration. Neurosci. Lett. 746, 135656 (2021).
Gadadhar, S. et al. Tubulin glycylation controls axonemal dynein activity, flagellar beat, and male fertility. Science 371, eabd4914 (2021).
Bobinnec, Y. et al. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol. 143, 1575–1589 (1998).
Ti, S. C., Alushin, G. M. & Kapoor, T. M. Human beta-tubulin isotypes can regulate microtubule protofilament number and stability. Dev. Cell 47, 175–190 (2018).
Chen, J. et al. α-Tubulin tail modifications regulate microtubule stability through selective effector recruitment, not changes in intrinsic polymer dynamics. Dev. Cell 56, 2016–2028 e4 (2021).
Hammond, J. W., Cai, D. & Verhey, K. J. Tubulin modifications and their cellular functions. Curr. Opin. Cell Biol. 20, 71–76 (2008).
Garnham, C. P. et al. Multivalent microtubule recognition by tubulin tyrosine ligase-like family glutamylases. Cell 161, 1112–1123 (2015).
Ramirez-Rios, S. et al. VASH1-SVBP and VASH2-SVBP generate different detyrosination profiles on microtubules. J. Cell Biol. 222, e202205096 (2023).
Mahalingan, K. K. et al. Structural basis for polyglutamate chain initiation and elongation by TTLL family enzymes. Nat. Struct. Mol. Biol. 27, 802–813 (2020).
Lukinavicius, G. et al. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat. Methods 11, 731–733 (2014).
Guidotti, N. & Fierz, B. Semisynthesis and reconstitution of nucleosomes carrying asymmetric histone modifications. Methods Mol. Biol. 2133, 263–291 (2020).
Fang, G. M. et al. Protein chemical synthesis by ligation of peptide hydrazides. Angew. Chem. Int. Ed. 50, 7645–7649 (2011).
Rappsilber, J., Mann, M. & Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2, 1896–1906 (2007).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Andreu-Carbo, M., Fernandes, S. & Aumeier, C. Two-color in vitro assay to visualize and quantify microtubule shaft dynamics. STAR Protoc 3, 101320 (2022).
Gasic, I., Boswell, S. A. & Mitchison, T. J. Tubulin mRNA stability is sensitive to change in microtubule dynamics caused by multiple physiological and toxic cues. PLoS Biol. 17, e3000225 (2019).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Acknowledgements
We thank J. Schaer, K. Kruse, E. Derivery and G. Grammbitter for discussions and comments on the manuscript. We thank B. Beckert and S. Nazarov of the Dubochet Center for Imaging for help with Cryo-EM data acquisition. We thank M. Pavlou from the Proteomics Core Facility at EPFL for helping with proteomic analysis of tubulin proteins. We would like to thank A.S. Jijumon and Satish Bodakuntla (Institut Curie) for technical support. S.F., M.C.V. and C.A. have been supported by the DIP of the Canton of Geneva, SNSF (31003A_182473), and the NCCR Chemical Biology. B.F. and P.G. thanks the NCCR Chemical Biology and EPFL for their support. C.J. is supported by the French National Research Agency (ANR) award ANR-17-CE13-0021 and the Fondation pour la Recherche Medicale (FRM) grant DEQ20170336756.
Author information
Authors and Affiliations
Contributions
B.F. conceived the project. B.F., P.G. and C.A. designed the experiments. E.E. and B.F. developed the tubulin semi-synthesis strategy with input from P.G., G.H. and C.A. E.E. performed peptide chemistry, tubulin expression and semi-synthesis, enzymatic assays and microtubule imaging, under supervision from B.F. N.A., N.G., T.M.R., L.R. and C.J. contributed reagents for tubulin expression and semi-synthesis. S.F. and M.C.V. performed cell experiments, microtubule imaging and analysis of in vitro tubulin dynamics, under supervision of C.A. G.H. and F.S. performed cryo-EM analysis, under supervision of P.G. B.F. and C.A. initially wrote the manuscript and all authors contributed to data analysis, discussion of results and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Ryoma Ohi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Purification of 1α1B/β3-tubulin dimers and CfaN-eSrt2a.
a) Left: An initial design of GFP-labelled tubulin carrying a N-terminal half of the Ava split-intein16 was transfected into RPE-1 cells. The protein was misfolded and did not incorporate in the microtubule network. Right: A GFP-labelled tubulin construct with its CTT replaced by the TTP-tag is functional and incorporated into the microtubule network in RPE-1 cells. Scale bars: 10 μm b) SDS-PAGE analysis of overexpression of tubulin 1α1B and β3-tubulin in High-Five insect cells at indicated time points, and Western blot analysis of the cell lysates using indicated antibodies. c) Overview and SDS-PAGE analysis of purification of semi-recombinant tubulin dimers by affinity purification of 1α1B containing dimers against the His-tag. This results in a pool of human 1α1B tubulin in combination with either human β3 or insect β-tubulin (βins). d) Overview of purification and SDS-PAGE analyses of fully recombinant, human tubulin dimers using tandem affinity chromatography via the His6-tag using a TALON column, followed by FLAG tag pulldown, resulting in pure 1α1B/β3-tubulin dimers. e) Deconvoluted mass spectrum of purified 1α1B/β3 in a time-of-flight measurement. f) Expression and purification of CfaN-eSrt2a (3).
Extended Data Fig. 2 Peptide synthesis of the fluorescein-tagged tubulin tail 2α1B-fluo.
a) RP-HPLC and ESI-MS analysis of 2α1B-Pra(C). b) A CuAAC reaction to attach the fluorescein moiety on 2α1B-Pra(C) was monitored over time by analytical HPLC and ESI-MS. Reaction scheme of the CuAAC reaction is given on the right side. c) In a one-pot reaction the N-terminal peptide fragment was oxidized with nitrite to obtain an azide. Thereafter, a thioester 2(N-MTG) was produced by addition of MTG. Given are the analytic RP-HPLC and ESI-MS of the starting reagent and final product. d) RP-HPLC and ESI-MS analysis of the purified product peptide 2α1B-fluo following native chemical ligation with 2(N-MTG) and 2α1B-fluo(C).
Extended Data Fig. 3 Characterization of tubulin semi-synthesis with fluorescein-tagged 2α1B-fluo.
a) Schematic overview of the tubulin tandem transamidation reaction to generate 5α1B-fluo. b) Initial optimization of tubulin tandem transamidation reaction using a mixed pool of tubulin dimers (Extended Data Fig. 1c). Left: Kinetics of the reaction, quantified via SDS-PAGE, fluorescence scan using fluorescein excitation, anti-His Western blot and quantification of the reaction yield. Middle: Varying the concentration of 3 using the indicated equivalents. Right: Varying the concentration of 2α1B-fluo using the indicated equivalents. c) ESI-MS analysis of tubulin from microtubules polymerized from 5α1B-fluo/β3. d) Tandem MS/MS spectrum of peptide (424–454) covering the ligation site and tubulin C-terminus tagged with fluorescein. Red: b+-ions, Blue: y+-ions. The lower case ‘c’ represents a carbamidomethylated cysteine residue and the lower case ‘x’ is the fluorescein linked amino acid. e) Schematic representation of tubulin semi-synthesis, followed by tubulin polymerization / depolymerization assay with ultracentrifugation (pellet – microtubule; supernatant – tubulin) to assess function of spliced, fluorescein-tagged tubulin dimers. f) SDS-PAGE analysis of tubulin semi-synthesis with subsequent polymerization/depolymerization assay. Letters indicating the lanes of the gel refer to the steps in scheme e). Reaction conditions: 100 μL volume, 5 μM 1α1B/β3, 25 μM 3, 125 μM 2α1B-fluo. Yield: 13%. In this assay the non-polymerized tubulin dimers were subjected to a second polymerization step with 10 μM Taxol. g) A second SDS-PAGE analysis of tubulin semi-synthesis this time with double the amount of tubulin as in f), Lanes are labelled referring to steps in scheme e). Reaction conditions: 100 μL volume, 10 μM 1α1B/β3, 25 μM 3, 125 μM 2α1B-fluo. Yield: 15 %. h) Analysis of α-tubulin K40ac by immunoblotting, comparing purified bovine brain tubulin and recombinant 1α1B, demonstrating high levels of K40ac in both purified and recombinant tubulins. i) Proteomic analysis of 1α1B acetylation. Blue bars indicate detected peptides, coloured residues indicate detected PTMs (note: Cys residues were carbamidomethylated using iodoacetamide before proteomic analysis). A majority of detected peptides show α-tubulin K40 acetylation, whereas other lysine sites were only very sporadically acetylated. j) Tandem MS/MS spectrum of a detected peptide from 1α1B, covering the K40 acetylation site followed by the introduced His6-tag, exhibiting K40 acetylation. Red: b+-ions, Blue: y+-ions. k) Tandem MS/MS spectrum of a detected peptide from 1α1B covering the K40 acetylation site followed by the introduced His6-tag, lacking any post-translational modification. Red: b+-ions, Blue: y+-ions.
Extended Data Fig. 4 Native chemical ligation to synthesize unmodified and polyglutamylated 2α1B peptide variants.
a) Analytical RP-HPLC and ESI-MS of 2(N-MPAA), and scheme of the following ligation reactions to generate full-length 2α1B, 2α1B-E1, 2α1B-E5 and 2α1B-E10. b-e) Native chemical ligation reactions to generate 2α1B, 2α1B-E1, 2α1B-E5 and 2α1B-E10. Top: Analytics of full-length fragments 2α1B(C), 2α1B-E1(C), 2α1B-E5(C), 2α1B-E10(C) via RP-HPLC and ESI-MS. Middle: Native chemical ligation between 2α1B(C), 2α1B-E1(C), 2α1B-E5(C) or 2α1B-E10(C) and 2(N-MPAA). Native chemical ligation of 2α1B-E1 appeared more difficult and displayed some by-products in the form of unprocessed hydrazide surrogate (*) and the hydrolysed thioester (+). Bottom: RP-HPLC and ESI-MS of purified 2α1B, 2α1B-E1, 2α1B-E5 and 2α1B-E10. f) Synthetic scheme to produce a detyrosinated form of unmodified or polyglutamylated 2α1B peptide. g) Analytics for detyrosinated, unmodified peptide 2α1B-∆Y via RP-HPLC and ESI-MS. h) Analytics for 2α1B-E10-∆Y via RP-HPLC and ESI-MS.
Extended Data Fig. 5 Semi-synthesis and characterization of unmodified and polyglutamylated tubulin variants.
a) Time-dependent reaction progress of semi-synthesis of 5α1B-E10, analysed via SDS-PAGE and Western blot using an anti-α-tubulin antibody. A quantification of the band intensities of the Western blot demonstrates the reaction progress. b) Polymerization/depolymerization of 5α1B-E5 after semi-synthesis. Left: Diagram of biochemical steps. Right: SDS-PAGE analysis and Western blot analysis using the indicated antibodies. The lanes (A to D) correspond to the reaction steps as labelled in the scheme on the left. c) Taxol-stabilized microtubules were made for every variant of 5α1B, 5α1B-E1, 5α1B-E5 and 5α1B-E10. For each semi-synthesis and polymerization, a SDS-PAGE analysis is given with Western blots detecting the different C-terminal tail modifications for α1B, α1B-E1, α1B-E5 and α1B-E10 by anti-Tyr against terminal tyrosine, GT335 against a monoglutamylated tail and pE against the polyglutamylated tail (>4 glutamates). Optimal conditions for this reaction are 10 μM 1α1B, 100 μM 3 and 150 μM 2 for 90 minutes (see Methods). Polymerization and pelleting remove the excess of 3. d-g) LC-MSToF (time-of-flight) of all given tubulin variants. Raw spectra on the left and corresponding deconvolution profile on the right of each tubulin variant of 5α1B including β-tubulin, (d) 5α1B-E1 (e), 5α1B-E5 (f) and 5α1B-E10 (g). Theoretical and observed masses are indicated. h) The tandem transamidation reaction generating 5α1B was analysed by LC-MSToF. Displayed are the total ion current chromatographs with a close-up on the tubulin elution peak for three different time points (0, 10 and 30 minutes). The deconvolved masses of the eluted tubulins are given on the right side. Only in the first time point a mass for the intermediate 4α1B could be observed, the deconvolved mass is displayed above.
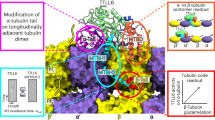
Extended Data Fig. 6 Structural characterization of tailless, unmodified and polyglutamylated tubulin variants.
a–c) FSC curves of the final cryo-EM maps of 1α1B, 5α1B, and 5α1B-E10, respectively. d-f) Top view of the cryo-EM of 1α1B, 5α1B, and 5α1B-E10 microtubules, respectively. The boxed region shows in ribbon the fitted structure of human tubulin (G-I). g-i) Front view of the cryo-EM of 1α1B, 5α1B, and 5α1B-E10, respectively. In the maps the structure of human tubulin is shown in ribbon representation. j-l) Cryo-EM maps at lower threshold levels of 1α1B, 5α1B, and 5α1B-E10, respectively, to show the difference in the additional density at the region of the CTT. Top view of a single protofilament is shown on the left while a side view on the right. Arrows point to the density region of the CTT.
Extended Data Fig. 7 Quantification of tubulin polyglutamylation and detyrosination in cells.
a) GFP-tubulin channel and overlay of all channels of cell shown in Fig. 3a. A 3D scan was performed for the region marked in Fig. 3a. Magenta represents pE IF intensity, cyan represents ∆Y intensity and white represents the colocalization region for both. Scale bars: 10 μm. b) Representative immunostaining of α-tubulin and ∆Y proving that the dotted pattern did not result from a fragmented network. Scale bars: 10 μm. c) Representative immunostaining of CRISPR/Cas9 knock-in GFP-Tubulin RPE-1 cells (green), antibody stained for detyrosination (∆Y, cyan) and acetylation (K40ac, magenta). Boxed region is magnified on the right. Scalebar: 10 μm. d) Representative immunostaining of CRISPR/Cas9 knock-in GFP-Tubulin RPE-1 cells (green), antibody stained for polyglutamylation (pE, cyan) and acetylation (K40ac, magenta). Boxed region is magnified on the right. Scalebar: 10 μm. e) Colocalization of pE and ∆Y was determined via Pearson correlation coefficient in RPE-1 cells. n = 3 independent experiments with 10 analysed cells per replicate. Mean with s.d. Statistics: one-way ANOVA. f) Western Blot analysis with indicated antibodies and quantification of polyglutamylation and detyrosination levels in the indicated different cell lines, PTM signal was normalizing to GAPDH and analys on the right. n = 3 independent experiments. Mean with s.d. Statistics: one-way ANOVA. g) Dot blot analysis of different amount of synthetic tubulin tail-IntN peptides, 2α1B-E10 and 2α1B-ΔY, using antibodies against polyglutamylated α-tubulin and detyrosinated α-tubulin respectively. Quantification of 2α1B-E10 and 2α1B-ΔY intensities with two individual experiments. h) Dot blot analysis of different amounts of synthetic tubulin tail-IntN peptides, 2α1B-E5 and 2α1B-E10, using anti-polyglutamylated α-tubulin antibody. Quantification of 2α1B-E5 and 2α1B-E10 intensities with three individual experiments. i) Dot blot analysis of different amount of synthetic tubulin tail-IntN peptides, 2α1B-E10-ΔY and 2α1B-E10, using anti-polyglutamylated α-tubulin antibody. Quantification of 2α1B-E10-ΔY and 2α1B-E10 intensities with two individual experiments.
Extended Data Fig. 8 Synthesis of polyglutamylated tubulin tail peptides and TIRF microscopy analysis of modified tubulins.
a) Analytical RP-HPLC and ESI-MS for peptides 2α1B-445HPG(C) (top), N-terminally azide functionalized fragments of 5 glutamates (middle) and N-terminally azide functionalized fragments of 10 glutamates (bottom). b) The CuAAC reaction to create 2α1B-E10(C), monitored over time by analytical RP-HPLC. c) Reaction scheme for the production of monoglutamylated peptide with a native isopeptide bond at the branch site. All purified products were used for native chemical ligation to complete the peptides 2α1B with various pE modifications (see Extended Data Fig. 4). d) Following polymerization with Taxol, the different microtubule variants were sedimented on a coverslip, stained and imaged with the different indicated antibodies against α-tubulin (anti-α, green), monoglutamylated α-tubulin (GT335, magenta), polyglutamylated α-tubulin (pE, magenta) and tyrosinated α-tubulin (anti-Tyr, blue). Displayed are tubulin variants from top to bottom: α1B, α1B-E1, α1B-E5 and α1B-E10. Scale bars are 10 μm. Note that β-tubulin is not glutamylated. e) Top: 5α1B microtubule visualized using 1 nM SiR-Tubulin17. Bottom: Kymograph of 5α1B microtubule dynamics.
Extended Data Fig. 9 Detyrosination kinetics of α1B/α1B-E5/α1B-E10 microtubule by vasohibin/SVBP.
a) Relative quantification of detyrosination by vasohibin-SVBP, showing individual repeats. Mean with SD. Statistics: one-way ANOVA, p < 0.05; p-values for α1B vs α1B-E5, α1B vs α1B-E10, α1B-E5 vs α1B-E10: 0.1, 0.28, 0.41 (0 min); 0.007, 0.0001, 0.001(5 min); 0.013, <0.0001, 0.008 (15 min); 0.009, <0.0001, <0.0001 (30 min). b) Western blot analysis of decreasing tyrosination levels upon vasohibin/SVBP treatment of indicated microtubule variants using antibodies against tyrosinated α-tubulin. c) Protein amount (determined by protein signal intensity in SDS-PAGE) and amount of tyrosinated α-tubulin (determined by Western blot) of microtubules used for the detyrosination assay. Plot below: normalized ratio of detyrosination over protein signal for α1B, α1B-E5 and α1B-E10. This demonstrates similar splicing efficiency and equal starting points for the assays. n = 4 independent experiments for α1B, n = 2 for α1B-E5 and n = 3 for α1B-E10. Mean with s.d. Statistics: one-way ANOVA. c) Relative quantification of detyrosination by vasohibin-SVBP, showing individual repeats. Mean with s.d. Statistics: one-way ANOVA. d) On the top, Dot blot analysis of different synthetic tubulin tail-IntN peptides, either unmodified (2α1B), detyrosinated (2α1B-ΔY) or polyglutamylated (2α1B-E5), using antibodies against tyrosinated α-tubulin, detyrosinated α-tubulin and polyglutamylated α-tubulin. On the bottom, Dot blot analysis of different synthetic tubulin tail-IntN peptides, detyrosinated (2α1B-ΔY), detyrosinated and polyglutamylated (2α1B-E10-ΔY), using antibodies against tyrosinated α-tubulin, detyrosinated α-tubulin and polyglutamylated α-tubulin. On the right, quantification of 2α1B-ΔY and 2α1B-E10-ΔY intensities for the anti-detyrosinated tubulin antibody signal (three individual experiments). Mean with s.d.
Extended Data Fig. 10 Overexpression or knockdown of pE writers control detyrosination levels in cells.
a) Representative immunostaining of RPE-1 cells overexpressing TTLL5/6 (yellow arrowhead) and a cell with low or no overexpression (right site). GFP: α-tubulin, magenta: pE, cyan: ∆Y. Boxed region is a zoom in highlighting that in low or non-overexpressing cells the level of polyglutamylation and detyrosination is much lower. Scale bars: 10 μm. b) Colocalization of pE and ∆Y in IF images (see Fig. 6a) was determined via Pearson correlation coefficient in RPE-1 cells which overexpressed TTLL5/6. n = 3 independent experiments with 10 analysed cells per replicate. Mean with s.d. Statistics: one-way ANOVA. c) Western Blot analysis underlying quantification in Fig. 6d. Analysis of PTM levels in TTLL5/6 overexpression RPE-1 cells compared to control RPE-1 cells using the indicated antibodies. d) Western Blot analysis underlying quantification in Fig. 6f. Analysis of PTM levels using indicated antibodies after knockdown of TTLLs (1, 5, 9, 11, 13) or control siRNA in RPE-1 cells. e) Western Blot analysis of PTM levels in TTLL5/6 overexpression U2OS cells (O/E TTLL5/6) compared to control U2OS cells (Ctrl) using the indicated antibodies. f) Quantification of e). Mean with s.d. of 4 independent experiments. Statistics: two-way ANOVA, two-factor without replication. The PTM signal was divided by the GAPDH signal, and the value for control U2OS (Ctr) cells was normalized to 1. g) Western Blot analysis of PTM levels using indicated antibodies after knockdown of TTLLs (1, 5, 9, 11, 13) (siRNA TTLLs) or control siRNA (Ctrl) in U2OS cells. h) Quantification of g). Mean with s.d. of 3 independent experiments. Statistics: two-way ANOVA, two-factor without replication.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3, Methods, and Tables 1 and 2.
Source data
Source Data Fig. 1
Western Blots and gels.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Western Blots and gels.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 5
Western Blots and gels.
Source Data Fig. 6
Statistical Source Data.
Source Data Fig. 6
Western Blots.
Source Data Extended Data Fig. 1
Western Blots and gels.
Source Data Extended Data Fig. 3
Western Blots and gels.
Source Data Extended Data Fig. 5
Western Blots and gels.
Source Data Extended Data Fig. 7
Statistical Source Data.
Source Data Extended Data Fig. 7
Western Blots.
Source Data Extended Data Fig. 9
Statistical Source Data.
Source Data Extended Data Fig. 9
Western Blots and gels.
Source Data Extended Data Fig. 10
Statistical Source Data.
Source Data Extended Data Fig. 10
Western Blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ebberink, E., Fernandes, S., Hatzopoulos, G. et al. Tubulin engineering by semi-synthesis reveals that polyglutamylation directs detyrosination. Nat. Chem. 15, 1179–1187 (2023). https://doi.org/10.1038/s41557-023-01228-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01228-8
This article is cited by
-
Structural basis for α-tubulin-specific and modification state-dependent glutamylation
Nature Chemical Biology (2024)
-
Microtubule-binding protein MAP1B regulates interstitial axon branching of cortical neurons via the tubulin tyrosination cycle
The EMBO Journal (2024)