Abstract
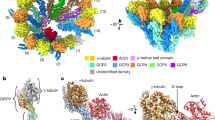
Microtubules have spatiotemporally complex posttranslational modification patterns. Tubulin tyrosine ligase-like (TTLL) enzymes introduce the most prevalent modifications on α-tubulin and β-tubulin. How TTLLs specialize for specific substrate recognition and ultimately modification-pattern generation is largely unknown. TTLL6, a glutamylase implicated in ciliopathies, preferentially modifies tubulin α-tails in microtubules. Cryo-electron microscopy, kinetic analysis and single-molecule biochemistry reveal an unprecedented quadrivalent recognition that ensures simultaneous readout of microtubule geometry and posttranslational modification status. By binding to a β-tubulin subunit, TTLL6 modifies the α-tail of the longitudinally adjacent tubulin dimer. Spanning two tubulin dimers along and across protofilaments (PFs) ensures fidelity of recognition of both the α-tail and the microtubule. Moreover, TTLL6 reads out and is stimulated by glutamylation of the β-tail of the laterally adjacent tubulin dimer, mediating crosstalk between α-tail and β-tail. This positive feedback loop can generate localized microtubule glutamylation patterns. Our work uncovers general principles that generate tubulin chemical and topographic complexity.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The TTLL6MTBH12:MT model has been deposited under accession PDB 8T42; the PDB model of TTLL6:MT from the composite map has been deposited under accession PDB 8U3Z. Maps were deposited at Electron Microscopy Data Bank with accession EMD-42884, B-factor sharpened map, EMD-41018 DeepEmhanced map, EMD-41090, composite map, all with accompanying raw half maps. The map of TTLL6 obtained by focused classification has accession EMD-41022. Motion-corrected micrographs are found under EMPIAR-11798. All plasmids and cell lines used in this study will be shared by the lead contact upon request. This paper does not use any original code. Source data are provided with this paper.
References
Roll-Mecak, A. The tubulin code in microtubule dynamics and information encoding. Dev. Cell 54, 7–20 (2020).
Bieling, P. et al. CLIP-170 tracks growing microtubule ends by dynamically recognizing composite EB1/tubulin-binding sites. J. Cell Biol. 183, 1223–1233 (2008).
Chen, J. et al. α-Tubulin tail modifications regulate microtubule stability through selective effector recruitment, not changes in intrinsic polymer dynamics. Dev. Cell 56, 2016–2028 (2021).
Hotta, T. et al. EML2-S constitutes a new class of proteins that recognizes and regulates the dynamics of tyrosinated microtubules. Curr. Biol. 32, 3898–3910 (2022).
Gundersen, G. G. & Bulinski, J. C. Selective stabilization of microtubules oriented toward the direction of cell migration. Proc. Natl Acad. Sci. USA 85, 5946–5950 (1988).
Gurland, G. & Gundersen, G. G. Stable, detyrosinated microtubules function to localize vimentin intermediate filaments in fibroblasts. J. Cell Biol. 131, 1275–1290 (1995).
Kerr, J. P. et al. Detyrosinated microtubules modulate mechanotransduction in heart and skeletal muscle. Nat. Commun. 6, 8526 (2015).
Palazzo, A. F., Eng, C. H., Schlaepfer, D. D., Marcantonio, E. E. & Gundersen, G. G. Localized stabilization of microtubules by integrin- and FAK-facilitated ρ signaling. Science 303, 836–839 (2004).
Robison, P. et al. Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science 352, aaf0659 (2016).
Lacroix, B. et al. Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J. Cell Biol. 189, 945–954 (2010).
Sharma, N. et al. Katanin regulates dynamics of microtubules and biogenesis of motile cilia. J. Cell Biol. 178, 1065–1079 (2007).
Szczesna, E. et al. Combinatorial and antagonistic effects of tubulin glutamylation and glycylation on katanin microtubule severing. Dev. Cell 57, 2497–2513 (2022).
Valenstein, M. L. & Roll-Mecak, A. Graded control of microtubule severing by tubulin glutamylation. Cell 164, 911–921 (2016).
Barisic, M. et al. Mitosis. Microtubule detyrosination guides chromosomes during mitosis. Science 348, 799–803 (2015).
Lessard, D. V. et al. Polyglutamylation of tubulin’s C-terminal tail controls pausing and motility of kinesin-3 family member KIF1A. J. Biol. Chem. 294, 6353–6363 (2019).
Sirajuddin, M., Rice, L. M. & Vale, R. D. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat. Cell Biol. 16, 335–344 (2014).
McKenney, R. J., Huynh, W., Vale, R. D. & Sirajuddin, M. Tyrosination of α-tubulin controls the initiation of processive dynein-dynactin motility. EMBO J. 35, 1175–1185 (2016).
Nirschl, J. J., Magiera, M. M., Lazarus, J. E., Janke, C. & Holzbaur, E. L. α-Tubulin tyrosination and CLIP-170 phosphorylation regulate the initiation of dynein-driven transport in neurons. Cell Rep. 14, 2637–2652 (2016).
van Dijk, J. et al. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol. Cell 26, 437–448 (2007).
Garnham, C. P. & Roll-Mecak, A. The chemical complexity of cellular microtubules: tubulin post-translational modification enzymes and their roles in tuning microtubule functions. Cytoskeleton (Hoboken) 69, 442–463 (2012).
Gundersen, G. G., Khawaja, S. & Bulinski, J. C. Generation of a stable, posttranslationally modified microtubule array is an early event in myogenic differentiation. J. Cell Biol. 109, 2275–2288 (1989).
Bodakuntla, S. et al. Tubulin polyglutamylation is a general traffic control mechanism in hippocampal neurons. J. Cell Sci. 133, jcs241802 (2020).
Magiera, M. M., Singh, P., Gadadhar, S. & Janke, C. Tubulin posttranslational modifications and emerging links to human disease. Cell 173, 1323–1327 (2018).
Karakaya, M. et al. Biallelic variant in AGTPBP1 causes infantile lower motor neuron degeneration and cerebellar atrophy. Am. J. Med. Genet. A 179, 1580–1584 (2019).
Maddirevula, S. et al. Autozygome and high throughput confirmation of disease genes candidacy. Genet. Med. 21, 736–742 (2019).
Shashi, V. et al. Loss of tubulin deglutamylase CCP1 causes infantile-onset neurodegeneration. EMBO J. 37, e100540 (2018).
Sheffer, R. et al. Biallelic variants in AGTPBP1, involved in tubulin deglutamylation, are associated with cerebellar degeneration and motor neuropathy. Eur. J. Hum. Genet. 27, 1419–1426 (2019).
Konno, A. et al. TTLL9−/− mice sperm flagella show shortening of doublet 7, reduction of doublet 5 polyglutamylation and a stall in beating. J. Cell Sci. 129, 2757–2766 (2016).
Pathak, N., Austin, C. A. & Drummond, I. A. Tubulin tyrosine ligase-like genes TTLL3 and TTLL6 maintain zebrafish cilia structure and motility. J. Biol. Chem. 286, 11685–11695 (2011).
Bosch Grau, M. et al. Tubulin glycylases and glutamylases have distinct functions in stabilization and motility of ependymal cilia. J. Cell Biol. 202, 441–451 (2013).
Ikegami, K., Sato, S., Nakamura, K., Ostrowski, L. E. & Setou, M. Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry. Proc. Natl Acad. Sci. USA 107, 10490–10495 (2010).
He, K. et al. Axoneme polyglutamylation regulated by Joubert syndrome protein ARL13B controls ciliary targeting of signaling molecules. Nat. Commun. 9, 3310 (2018).
Hong, S. R. et al. Spatiotemporal manipulation of ciliary glutamylation reveals its roles in intraciliary trafficking and Hedgehog signaling. Nat. Commun. 9, 1732 (2018).
Kubo, T. et al. A conserved flagella-associated protein in Chlamydomonas, FAP234, is essential for axonemal localization of tubulin polyglutamylase TTLL9. Mol. Biol. Cell 25, 107–117 (2014).
Lee, J. E. et al. CEP41 is mutated in Joubert syndrome and is required for tubulin glutamylation at the cilium. Nat. Genet. 44, 193–199 (2012).
Bompard, G. et al. CSAP acts as a regulator of TTLL-mediated microtubule glutamylation. Cell Rep. 25, 2866–2877 (2018).
Backer, C. B., Gutzman, J. H., Pearson, C. G. & Cheeseman, I. M. CSAP localizes to polyglutamylated microtubules and promotes proper cilia function and zebrafish development. Mol. Biol. Cell 23, 2122–2130 (2012).
Mullen, R. J., Eicher, E. M. & Sidman, R. L. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc. Natl Acad. Sci. USA 73, 208–212 (1976).
Garnham, C. P. et al. Multivalent microtubule recognition by tubulin tyrosine ligase-like family glutamylases. Cell 161, 1112–1123 (2015).
Janke, C. et al. Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science 308, 1758–1762 (2005).
Mahalingan, K. K. et al. Structural basis for polyglutamate chain initiation and elongation by TTLL family enzymes. Nat. Struct. Mol. Biol. 27, 802–813 (2020).
Mukai, M. et al. Recombinant mammalian tubulin polyglutamylase TTLL7 performs both initiation and elongation of polyglutamylation on β-tubulin through a random sequential pathway. Biochemistry 48, 1084–1093 (2009).
Bonnet, C. et al. Differential binding regulation of microtubule-associated proteins MAP1A, MAP1B, and MAP2 by tubulin polyglutamylation. J. Biol. Chem. 276, 12839–12848 (2001).
Boucher, D., Larcher, J. C., Gros, F. & Denoulet, P. Polyglutamylation of tubulin as a progressive regulator of in vitro interactions between the microtubule-associated protein τ and tubulin. Biochemistry 33, 12471–12477 (1994).
Genova, M. et al. Tubulin polyglutamylation differentially regulates microtubule-interacting proteins. EMBO J. 42, e112101 (2023).
Kubo, T., Yanagisawa, H. A., Yagi, T., Hirono, M. & Kamiya, R. Tubulin polyglutamylation regulates axonemal motility by modulating activities of inner-arm dyneins. Curr. Biol. 20, 441–445 (2010).
Suryavanshi, S. et al. Tubulin glutamylation regulates ciliary motility by altering inner dynein arm activity. Curr. Biol. 20, 435–440 (2010).
Zhong, Y. et al. XBP1 variant 1 promotes mitosis of cancer cells involving upregulation of the polyglutamylase TTLL6. Hum. Mol. Genet. 31, 2639–2654 (2022).
Miller, K. E. & Heald, R. Glutamylation of Nap1 modulates histone H1 dynamics and chromosome condensation in Xenopus. J. Cell Biol. 209, 211–220 (2015).
Sun, X. et al. Loss of RPGR glutamylation underlies the pathogenic mechanism of retinal dystrophy caused by TTLL5 mutations. Proc. Natl Acad. Sci. USA 113, E2925–E2934 (2016).
Xia, P. et al. Glutamylation of the DNA sensor cGAS regulates its binding and synthase activity in antiviral immunity. Nat. Immunol. 17, 369–378 (2016).
Vemu, A., Garnham, C. P., Lee, D. Y. & Roll-Mecak, A. Generation of differentially modified microtubules using in vitro enzymatic approaches. Methods Enzymol. 540, 149–166 (2014).
Vemu, A., Atherton, J., Spector, J. O., Moores, C. A. & Roll-Mecak, A. Tubulin isoform composition tunes microtubule dynamics. Mol. Biol. Cell 28, 3564–3572 (2017).
Debs, G. E. et al. Dynamic and asymmetric fluctuations in the microtubule wall captured by high-resolution cryoelectron microscopy. Proc. Natl Acad. Sci. USA 117, 16976–16984 (2020).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Zehr, E. A. & Roll-Mecak, A. Cryo-EM structures of human α1B/βI+βIVb microtubules shed light on isoform specific assembly. Preprint at bioRxiv https://doi.org/10.1101/2023.12.01.569594 (2023).
Kellogg, E. H. et al. Insights into the distinct mechanisms of action of taxane and non-taxane microtubule stabilizers from cryo-EM structures. J. Mol. Biol. 429, 633–646 (2017).
Sui, H. & Downing, K. H. Structural basis of interprotofilament interaction and lateral deformation of microtubules. Structure 18, 1022–1031 (2010).
Garnham, C. P., Yu, I., Li, Y. & Roll-Mecak, A. Crystal structure of tubulin tyrosine ligase-like 3 reveals essential architectural elements unique to tubulin monoglycylases. Proc. Natl Acad. Sci. USA 114, 6545–6550 (2017).
Ikegami, K. et al. TTLL7 is a mammalian β-tubulin polyglutamylase required for growth of MAP2-positive neurites. J. Biol. Chem. 281, 30707–30716 (2006).
Abad, M. A. et al. Structural basis for microtubule recognition by the human kinetochore Ska complex. Nat. Commun. 5, 2964 (2014).
Legal, T., Zou, J., Sochaj, A., Rappsilber, J. & Welburn, J. P. Molecular architecture of the Dam1 complex-microtubule interaction. Open Biol. 6, 150237 (2016).
Manka, S. W. & Moores, C. A. The role of tubulin-tubulin lattice contacts in the mechanism of microtubule dynamic instability. Nat. Struct. Mol. Biol. 25, 607–615 (2018).
Maurer, S. P., Fourniol, F. J., Bohner, G., Moores, C. A. & Surrey, T. EBs recognize a nucleotide-dependent structural cap at growing microtubule ends. Cell 149, 371–382 (2012).
Zhang, R., Alushin, G. M., Brown, A. & Nogales, E. Mechanistic origin of microtubule dynamic instability and its modulation by EB proteins. Cell 162, 849–859 (2015).
Roll-Mecak, A. How cells exploit tubulin diversity to build functional cellular microtubule mosaics. Curr. Opin. Cell Biol. 56, 102–108 (2019).
Redeker, V. et al. Mutations of tubulin glycylation sites reveal cross-talk between the C termini of α- and β-tubulin and affect the ciliary matrix in Tetrahymena. J. Biol. Chem. 280, 596–606 (2005).
Ebberink, E. et al. Tubulin engineering by semisynthesis reveals that polyglutamylation directs detyrosination. Nat. Chem. 15, 1179–1187 (2023).
Latham, J. A. & Dent, S. Y. Cross-regulation of histone modifications. Nat. Struct. Mol. Biol. 14, 1017–1024 (2007).
Suganuma, T. & Workman, J. L. Crosstalk among histone modifications. Cell 135, 604–607 (2008).
Magiera, M. M. et al. Excessive tubulin polyglutamylation causes neurodegeneration and perturbs neuronal transport. EMBO J. 37, e100440 (2018).
Zempel, H. et al. Amyloid-β oligomers induce synaptic damage via τ-dependent microtubule severing by TTLL6 and spastin. EMBO J. 32, 2920–2937 (2013).
Zhang, F. et al. Posttranslational modifications of α-tubulin in Alzheimer disease. Transl. Neurodegener. 4, 9 (2015).
Gadau, S. D. Morphological and quantitative analysis on α-tubulin modifications in glioblastoma cells. Neurosci. Lett. 687, 111–118 (2018).
Mialhe, A. et al. Tubulin detyrosination is a frequent occurrence in breast cancers of poor prognosis. Cancer Res. 61, 5024–5027 (2001).
Soucek, K. et al. Normal and prostate cancer cells display distinct molecular profiles of α-tubulin posttranslational modifications. Prostate 66, 954–965 (2006).
Jurrus, E. et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 27, 112–128 (2018).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Lander, G. C. et al. Appion: an integrated, database-driven pipeline to facilitate EM image processing. J. Struct. Biol. 166, 95–102 (2009).
Zhang, K. GCTF: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Cook, A. D., Manka, S. W., Wang, S., Moores, C. A. & Atherton, J. A microtubule RELION-based pipeline for cryo-EM image processing. J. Struct. Biol. 209, 107402 (2020).
Vilas, J. L. et al. MonoRes: automatic and accurate estimation of local resolution for electron microscopy maps. Structure 26, 337–344 (2018).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D Struct. Biol. 74, 531–544 (2018).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Morin, A. et al. Collaboration gets the most out of software. eLife 2, e01456 (2013).
Barad, B. A. et al. EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nat. Methods 12, 943–946 (2015).
Sobolev, O. V. et al. A global Ramachandran score identifies protein structures with unlikely stereochemistry. Structure 28, 1249–1258 (2020).
He, S. & Scheres, S. H. W. Helical reconstruction in RELION. J. Struct. Biol. 198, 163–176 (2017).
Perez-Iratxeta, C. & Andrade-Navarro, M. A. K2D2: estimation of protein secondary structure from circular dichroism spectra. BMC Struct. Biol. 8, 25 (2008).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Ziolkowska, N. E. & Roll-Mecak, A. In vitro microtubule severing assays. Methods Mol. Biol. 1046, 323–334 (2013).
Banerjee, A., Bovenzi, F. A. & Bane, S. L. High-resolution separation of tubulin monomers on polyacrylamide minigels. Anal. Biochem. 402, 194–196 (2010).
Theile, C. S. et al. Site-specific N-terminal labeling of proteins using sortase-mediated reactions. Nat. Protoc. 8, 1800–1807 (2013).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Ramirez-Aportela, E. et al. Automatic local resolution-based sharpening of cryo-EM maps. Bioinformatics 36, 765–772 (2020).
Acknowledgements
We thank D.-Y. Lee from the Biochemistry Core (National Heart, Lung and Blood Institute (NHLBI)) for mass spectrometry help, J. Spector (National Institute of Neurological Disorders and Stroke (NINDS)) for microscopy help, D. Hoover (National Institutes of Health (NIH)) for software installation help, G.E. Debs (Yale University) and C.V. Sindelar (Yale University) for advice with PF refinement and modifying the procedure to perform two-PF refinement. Image processing was performed on the Biowulf cluster maintained by the High Performing Computation group at the NIH. G.C.L. (Scripps Research) is supported by NIH NS095892. A.R.-M. is supported by the intramural programs of NINDS and NHLBI.
Author information
Authors and Affiliations
Contributions
K.K.M. built the TTLL6 model and performed all functional assays and structural analyses. D.A.G. collected cryo-EM data. Y.L. obtained mass spectrometry data. D.A.G. and G.C.L. obtained initial C1 reconstruction. E.A.Z. performed MT reconstruction and PF refinement, obtained high-resolution reconstruction and refined atomic models. K.K.M, E.A.Z. and A.R.M. analyzed structures. K.K.M. and A.R.-M. interpreted functional data and wrote the manuscript with contributions from E.A.Z. All authors read, edited and approved the manuscript. A.R.-M. initiated, coordinated and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Anastassis Perrakis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cryo-EM image processing.
a. Initial image processing was performed in RELION83. In total, 2,771 movies were motion corrected and summed98; high-quality micrographs were selected based on CTF estimation80 and visual inspection for manual picking of microtubule segments. b. Representative cryo-EM micrograph from one of 2,771 movies of microtubules decorated with TTLL6. Decoration is highly heterogeneous. Scale bar, 25 nm. c. Initial reconstruction of microtubule decorated with TTLL6 using MiRP81. Microtubule segments were sorted based on protofilament number; most common rotation angle, x/y shifts and seam positions were assigned to each microtubule segment. d. C1 reconstruction of the TTLL6 bound microtubule. TTLL6, gold, binds every 80 Å, highlighted by the double arrow, along the microtubule lattice, blue. e. Protofilament refinement54 improved map quality for the microtubule and MTBH1-2 in TTLL6. For each microtubule segment all but the signal from two adjacent protofilaments was substracted to generate a two protofilament stack, followed by refinement of particle coordinates. f. Local resolution estimates show the map reaching 3 Å resolution in the microtubule and MTBH1-2 regions and poorly resolved density for the rest of TTLL6. MTBH1-2 is highlighted by a dashed rectangle. Local resolution was determined via Monores82 followed by LocalDeblur99. g. Two protofilament, 2PF, refinement protocol improved definition of the C-terminus of MTBH2 in comparison to a protocol where each particle contained signal for just one protofilament, 1PF. h. Focused classification of TTLL6 improved its definition. Refinement of the minor class yielded a TTLL6 map with local resolution estimates ranging from ∼5 Å to 14 Å. i Fourier shell correlation (FSC) curves for each microtubule reconstruction depict their nominal resolution. FSC estimates were calculated at 0.143 criterion. Resolution for the microtubule lattice improved from 3.8 Å, MiRP protocol, to 3.6 Å, two protofilament refinement protocol.
Extended Data Fig. 2 MTBH1-2 is highly flexible and binds only to microtubules and not soluble tubulin.
a. Ribbon representation of TTLL6 in the conformation it adopts in complex with the microtubule (domains colored as in Fig. 1) superposed on the X-ray crystal structures of TTLL6 bound to ATP (light gray, PDB 6VZT; ref. 41) or bound to a di-Glu elongation analog (dark gray, PDB 6VZU; ref. 41). A part of the MTBH1-2 was not resolved in the X-ray structures. The unresolved regions are shown as dotted lines. The MTBH1-2 in the TTLL6 X-ray structures, highlighted by a cyan ellipse, is in a different orientation than that in the cryo-EM structure in complex with the microtubule. b. Circular dichroism spectra of recombinantly expressed and purified TTLL6 MTBH1-2 show its secondary structure is predominantly α-helical. c. Binding of TTLL6 MTBH1-2 to taxol-stabilized microtubules assembled from porcine brain tubulin. Kd ∼3.7 μM. Error bars, S.E.M (n = 2). d. Gel filtration analysis of recombinant TTLL6 MTBH1-2 and soluble αβ-tubulin mixture showing that the two proteins elute separately on a Superdex-200 analytical gel-filtration column (Methods).
Extended Data Fig. 3 TTLL6 binding compacts the microtubule lattice and regularizes the microtubule wall.
a. Histogram of rotation angles (φ angle) between adjacent protofilaments of undecorated microtubules shows two peaks for non-seam 23.9°, major, and 30.7°, minor; for seam 23.7°, major, 30.7°, minor. These numbers deviate from a symmetric protofilament geometry, φ = 360°/14 protofilaments = 25.7°. b. Histogram of rotation angles (φ angle) between adjacent protofilaments of TTLL6-decorated microtubules shows one peak for non-seam at 25.4° and for the seam at 25.5°, These numbers are close to a symmetric protofilament geometry, φ = 360°/14 protofilaments = 25.7°. c. Atomic models of undecorated, gray, and TTLL6 decorated, red, protofilaments aligned at the minus end on the α1-tubulin subunit show TTLL6-induced structural changes in the microtubule lattice. d. Tubulin dimer repeat distances for undecorated and TTLL6-decorated microtubules obtained in Relion92 by refining the helical rise for 3 independent reconstructions obtained by splitting the data into three datasets, each containing one-third of the data (Methods). e. Tubulin dimer repeat distances obtained from fitting atomic models in 3 independent C1 and symmetrized undecorated and TTLL6-decorated microtubule reconstructions each obtained from one-third of the data (Methods).
Extended Data Fig. 4 MTBH1-2 destabilizes microtubules.
a–d. Representative cryo-EM images from one of two independent experiments showing microtubules alone or in the presence of excess MTBH1-2. MTBH1-2 at 5 μM with 1 μM GMPCPP-stabilized microtubules polymerized from porcine brain tubulin (a), 1 μM GMPCPP-stabilized microtubules polymerized from unmodified human tubulin affinity purified from tSA201 cells52 (b), 1 μM taxol-stabilized microtubules polymerized from porcine brain tubulin (c) and taxol-stabilized microtubules polymerized from unmodified human tubulin (d). Scale bar, 30 nm.
Extended Data Fig. 5 MTBD and MTBH1-2 optimally position the TTLL6 core to interact with the negatively charged α-tubulin tail through a groove lined with positively charged residues critical for activity.
a. View of TTLL6 in complex with the microtubule showing the proximity of the C-terminal tail of α-tubulin to the active site of TTLL6. TTLL6 surface is color-coded for electrostatic potential. The electrostatic potential was calculated using the Poisson Boltzmann Solver77. The α-tubulin tail, shown in green, for which no structural data are available was modeled to maximize side chain interactions without violating stereochemical constraints. ATP and the di-glutamate in the active site are shown in ball-and-stick. The di-glutamate position is based on the X-ray crystal structure of TTLL6 in complex with the α-elongation analog (PDB 6VZU; ref. 41). A groove lined with positively charged residues leads to the active site of TTLL6. We hypothesize that these cationic residues anchor electronegative side chains in the α-tubulin tail, while the hydrophobic groove interacts with the tubulin tail backbone. b,c. Normalized glutamylation activity of structure-guided TTLL6 mutants in the proposed α-tubulin tail binding groove with taxol-stabilized microtubules (b) and isolated α1B(-Y) peptide (c). Error bars, S.E.M. (n = 4). ****p < 0.0001 as determined by two-tailed t-test.
Extended Data Fig. 6 Identification of TTLL6 MTBH1-2-microtubule interaction sites using cross-linking coupled with tandem mass spectrometry.
a. Chemical reaction showing the cross-linking of primary amine and carboxylic acid in the presence of 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and sulfo N-hydroxysuccinimide (sulfo-NHS). b,c. Representative SDS-PAGE gels from one of two independent experiments showing the cross-linking of MTBH1-2 to microtubules (b) but not to soluble tubulin (c). The cross-linked products are outlined with a magenta rectangle. M, mock. d–h. MS/MS sequencing of the cross-linked products. Peptides were fragmented using EThcD decision tree method, so majority of the MS/MS fragments are c- and z-ions, with a small portion of b- and y-ions. The cross-linked product contains 2 peptides. The sequences of the peptides are shown in the spectrum and labeled as (α) or (β) peptides. When a MS/MS fragment contains cross-linked (α) and (β) peptides, it is labeled as normal c- or z-ion. When a fragment is from (α) or (β) peptides alone, α or β is then included in the labeling of that ion, such as [z12α+1] or c3β. The cross-link sites are highlighted in red. Possible cross-linked residues are underlined when it is not possible to locate the precise site.
Extended Data Fig. 7 Distance between MTBH2 and β-tubulin tails on the microtubule and mass spectra showing subtilisin and subtilisin mediated proteolytic removal of β-tubulin tails from unmodified human microtubules.
a. Schematic showing the distance between the start of the β-tubulin tail (yellow star) and the MTBH2 (cyan star). The disposition of the β-tubulin tail (its length and position as it emerges from the tubulin body) is such that the MTBH2 is able to interact only with the β-tail from the lateral tubulin dimer because the most C-terminal glutamate that cross-links to the MTBH2 lysine cannot reach closer than ∼30 Å from the C-terminus of MTBH2, even when we assume that the β-tail adopts a completely extended random coil conformation (3.8 Å Cα to Cα distance) which would give it the greatest span (the distance between the start of the β-tail and the MTBH2 on the same tubulin dimer is ∼75 Å). Even the most C-terminal glutamate residue in the β-tail of the same dimer on which TTLL6 sits cannot reach closer than ∼18 Å from MTBH2. In contrast, the Cα of the terminal Lys residue of TTLL6 MTBH2 is positioned ∼9 Å away from the last β-tubulin residue visible in our structure (D427, and the start of the flexible β-tubulin tail) that belongs to the laterally adjacent β-tubulin. b,c. LC-MS spectra of wild-type (b) and subtilisin (c) treated unmodified human microtubules used in Fig. 4 (Methods). The tubulin isotypes and the different β-tubulin species after subtilisin treatment are indicated.
Extended Data Fig. 8 Microtubules missing their β-tubulin tails are poor substrates for TTLL6.
a,b. LC-MS spectra of intact wild-type microtubules at times 0 h (a) and 2 h (b) after incubation with TTLL6. Tubulin isotypes and glutamate numbers are indicated on top. c,d. LC-MS spectra of αβΔ-tail microtubules at times 0 h (c) and 2 h (d) after incubation with TTLL6 (Methods). Spectra labeled as in a.
Extended Data Fig. 9 MTBH1-2 promotes tubulin polymerization and mutation of residues in the three cationic clusters impairs this activity.
a. Quantification of tubulin polymerization over time in the absence or presence of MTBH1-2 and the structure-guided MTBH1-2 mutants. b–f. Representative interference reflection microscopy images from one of two independent experiments of reactions containing 10 μM porcine tubulin without MTBH1-2 (b) or with 2.5 μM MTBH1-2 (c), 2.5 μM MTBH1-2 (K490A/K494A) (d), 25 μM MTBH1-2 (K490A/K494A) (e) and 25 μM MTBH1-2 (K490A/K494A/K498A/K502A) (f). Scale bar, 25 μm.
Extended Data Fig. 10 Single molecule characterization of Atto488-TTLL6 interaction with the microtubule.
a. Distribution of surface-bound single Atto488 TTLL6 over time. The number of surface-bound molecules decays exponentially as the fluorescence is bleached. An exponential decay fit (red line) yields a bleaching time of 12.5 s. b. One-step photobleaching trace of immobilized Atto488-TTLL6 consistent with the molecule existing as a monomer over the exposure time (50 ms). c. Initial fluorescence intensity distribution of Atto488-TTLL6 molecules immobilized on glass showing a monodisperse profile. d. Representative kymograph of single Atto488-TTLL6 on taxol-stabilized brain microtubules (left). Distribution of residence times of Atto488-TTLL6 with taxol-stabilized brain microtubules. The mean residence time (τ) was obtained by fitting an exponential curve to the histogram and correcting for photobleaching (R2 = 0.95, n = 791 binding events from 6 independent experiments). e. Sequence alignment of the MTBH1-2 from Mus musculus TTLL6 and TTLL13. Positively charged basic residues in the three clusters are marked with blue asterisks on top.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2, and Figs. 1–3.
Supplementary Data 1
Supporting data for Supplementary Fig. 3.
Supplementary Data 2
Oligonucleotide sequences for generating TTLL6 mutants.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
About this article
Cite this article
Mahalingan, K.K., Grotjahn, D.A., Li, Y. et al. Structural basis for α-tubulin-specific and modification state-dependent glutamylation. Nat Chem Biol (2024). https://doi.org/10.1038/s41589-024-01599-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41589-024-01599-0